Input interpretation
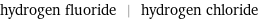
hydrogen fluoride | hydrogen chloride
Chemical names and formulas
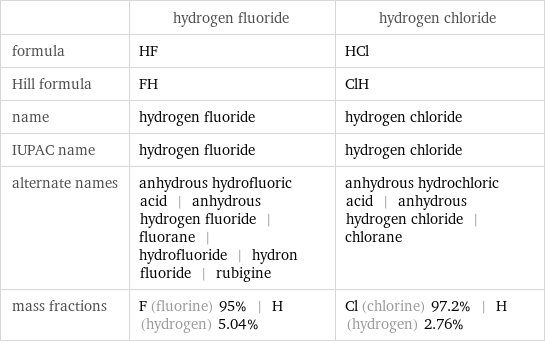
| hydrogen fluoride | hydrogen chloride formula | HF | HCl Hill formula | FH | ClH name | hydrogen fluoride | hydrogen chloride IUPAC name | hydrogen fluoride | hydrogen chloride alternate names | anhydrous hydrofluoric acid | anhydrous hydrogen fluoride | fluorane | hydrofluoride | hydron fluoride | rubigine | anhydrous hydrochloric acid | anhydrous hydrogen chloride | chlorane mass fractions | F (fluorine) 95% | H (hydrogen) 5.04% | Cl (chlorine) 97.2% | H (hydrogen) 2.76%
Structure diagrams

Structure diagrams
3D structure
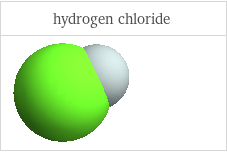
3D structure
Basic properties
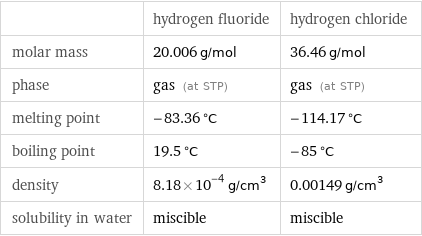
| hydrogen fluoride | hydrogen chloride molar mass | 20.006 g/mol | 36.46 g/mol phase | gas (at STP) | gas (at STP) melting point | -83.36 °C | -114.17 °C boiling point | 19.5 °C | -85 °C density | 8.18×10^-4 g/cm^3 | 0.00149 g/cm^3 solubility in water | miscible | miscible
Units

Gas properties (at STP)
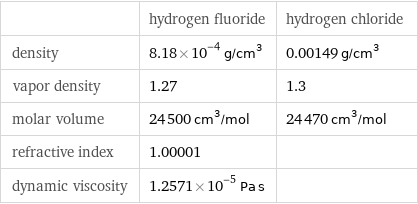
| hydrogen fluoride | hydrogen chloride density | 8.18×10^-4 g/cm^3 | 0.00149 g/cm^3 vapor density | 1.27 | 1.3 molar volume | 24500 cm^3/mol | 24470 cm^3/mol refractive index | 1.00001 | dynamic viscosity | 1.2571×10^-5 Pa s |
Units
Liquid properties

| hydrogen chloride sound speed | 1066 km/h
Units

Thermodynamic properties
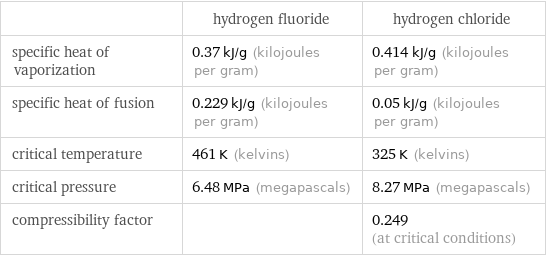
| hydrogen fluoride | hydrogen chloride specific heat of vaporization | 0.37 kJ/g (kilojoules per gram) | 0.414 kJ/g (kilojoules per gram) specific heat of fusion | 0.229 kJ/g (kilojoules per gram) | 0.05 kJ/g (kilojoules per gram) critical temperature | 461 K (kelvins) | 325 K (kelvins) critical pressure | 6.48 MPa (megapascals) | 8.27 MPa (megapascals) compressibility factor | | 0.249 (at critical conditions)