Input interpretation
calcium cation | beryllium cation | barium cation
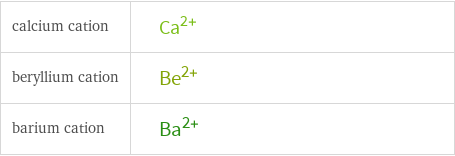
Structure diagrams
Structure diagrams
General properties
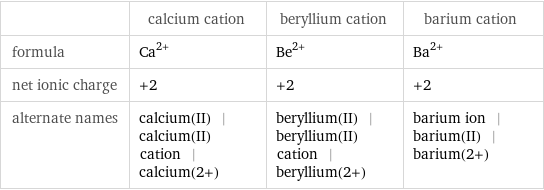
| calcium cation | beryllium cation | barium cation formula | Ca^(2+) | Be^(2+) | Ba^(2+) net ionic charge | +2 | +2 | +2 alternate names | calcium(II) | calcium(II) cation | calcium(2+) | beryllium(II) | beryllium(II) cation | beryllium(2+) | barium ion | barium(II) | barium(2+)
Ionic radii
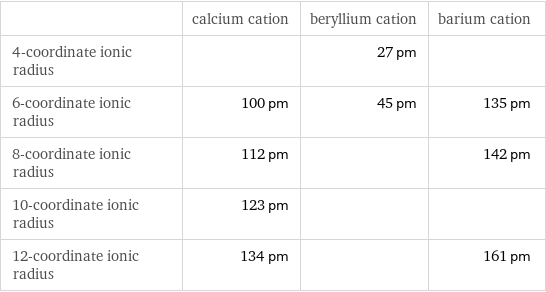
| calcium cation | beryllium cation | barium cation 4-coordinate ionic radius | | 27 pm | 6-coordinate ionic radius | 100 pm | 45 pm | 135 pm 8-coordinate ionic radius | 112 pm | | 142 pm 10-coordinate ionic radius | 123 pm | | 12-coordinate ionic radius | 134 pm | | 161 pm
Units

Other properties
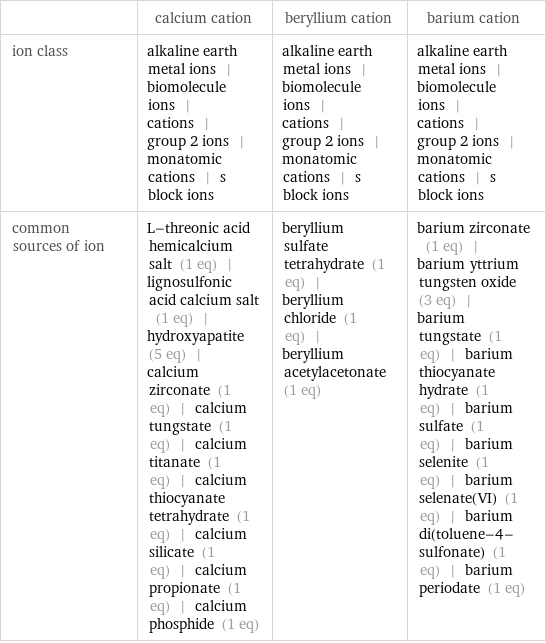
| calcium cation | beryllium cation | barium cation ion class | alkaline earth metal ions | biomolecule ions | cations | group 2 ions | monatomic cations | s block ions | alkaline earth metal ions | biomolecule ions | cations | group 2 ions | monatomic cations | s block ions | alkaline earth metal ions | biomolecule ions | cations | group 2 ions | monatomic cations | s block ions common sources of ion | L-threonic acid hemicalcium salt (1 eq) | lignosulfonic acid calcium salt (1 eq) | hydroxyapatite (5 eq) | calcium zirconate (1 eq) | calcium tungstate (1 eq) | calcium titanate (1 eq) | calcium thiocyanate tetrahydrate (1 eq) | calcium silicate (1 eq) | calcium propionate (1 eq) | calcium phosphide (1 eq) | beryllium sulfate tetrahydrate (1 eq) | beryllium chloride (1 eq) | beryllium acetylacetonate (1 eq) | barium zirconate (1 eq) | barium yttrium tungsten oxide (3 eq) | barium tungstate (1 eq) | barium thiocyanate hydrate (1 eq) | barium sulfate (1 eq) | barium selenite (1 eq) | barium selenate(VI) (1 eq) | barium di(toluene-4-sulfonate) (1 eq) | barium periodate (1 eq)