Input interpretation
titanium(III) oxide
Chemical names and formulas

formula | Ti_2O_3 Hill formula | O_3Ti_2 name | titanium(III) oxide alternate names | dititanium trioxide | keto-(ketotitaniooxy)titanium | oxo-(oxotitaniooxy)titanium | titanium oxide mass fractions | O (oxygen) 33.4% | Ti (titanium) 66.6%
Structure diagram
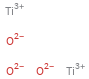
Structure diagram
Basic properties
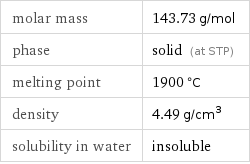
molar mass | 143.73 g/mol phase | solid (at STP) melting point | 1900 °C density | 4.49 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)

density | 4.49 g/cm^3
Units

Thermodynamic properties
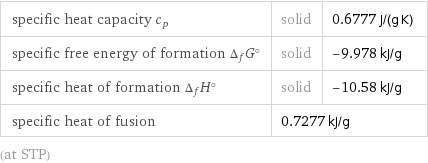
specific heat capacity c_p | solid | 0.6777 J/(g K) specific free energy of formation Δ_fG° | solid | -9.978 kJ/g specific heat of formation Δ_fH° | solid | -10.58 kJ/g specific heat of fusion | 0.7277 kJ/g | (at STP)
Units
Chemical identifiers
![CAS number | 1344-54-3 SMILES identifier | [O-2].[O-2].[O-2].[Ti+3].[Ti+3]](../image_source/f68ef7ccaa3654a791cba18684c8b184.png)
CAS number | 1344-54-3 SMILES identifier | [O-2].[O-2].[O-2].[Ti+3].[Ti+3]