Input interpretation
lithium hydroxide monohydrate strontium
Basic properties
![molar mass | 163.6 g/mol formula | H_5LiO_4Sr empirical formula | Sr_O_4Li_H_5 SMILES identifier | [Li+].O.[OH-].[OH-].[OH-].[Sr+2] InChI identifier | InChI=1/Li.4H2O.Sr/h;4*1H2;/q+1;;;;;+2/p-3/fLi.H2O.3HO.Sr/h;;3*1h;/qm;;3*-1;m InChI key | CXDODUXXDSRBGD-UHFFFAOYSA-K](../image_source/8e3ecc1759b9296a02acc58caee1a776.png)
molar mass | 163.6 g/mol formula | H_5LiO_4Sr empirical formula | Sr_O_4Li_H_5 SMILES identifier | [Li+].O.[OH-].[OH-].[OH-].[Sr+2] InChI identifier | InChI=1/Li.4H2O.Sr/h;4*1H2;/q+1;;;;;+2/p-3/fLi.H2O.3HO.Sr/h;;3*1h;/qm;;3*-1;m InChI key | CXDODUXXDSRBGD-UHFFFAOYSA-K
Structure diagram
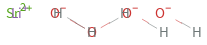
Structure diagram
Quantitative molecular descriptors
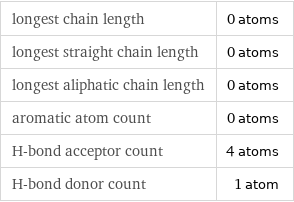
longest chain length | 0 atoms longest straight chain length | 0 atoms longest aliphatic chain length | 0 atoms aromatic atom count | 0 atoms H-bond acceptor count | 4 atoms H-bond donor count | 1 atom
Elemental composition
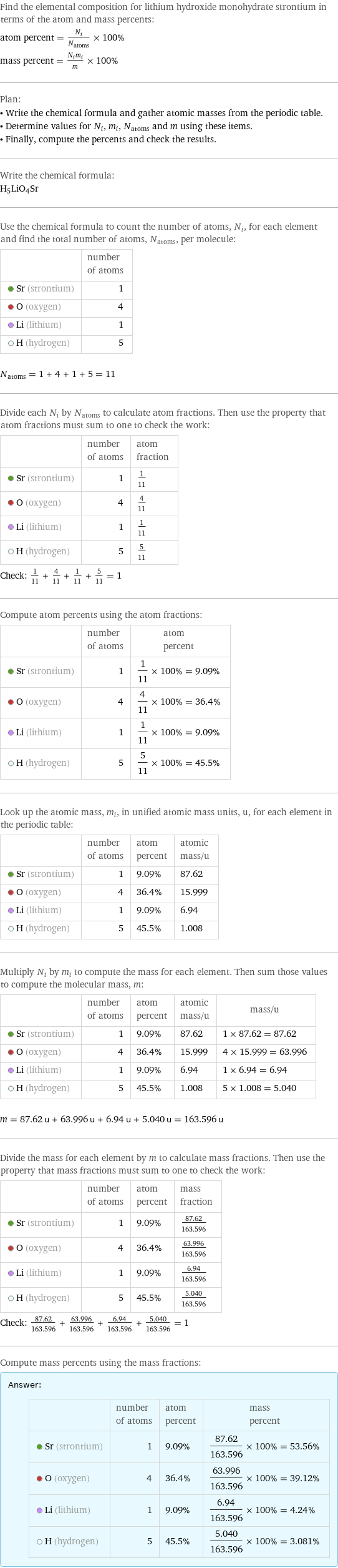
Find the elemental composition for lithium hydroxide monohydrate strontium in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: H_5LiO_4Sr Use the chemical formula to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms, per molecule: | number of atoms Sr (strontium) | 1 O (oxygen) | 4 Li (lithium) | 1 H (hydrogen) | 5 N_atoms = 1 + 4 + 1 + 5 = 11 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction Sr (strontium) | 1 | 1/11 O (oxygen) | 4 | 4/11 Li (lithium) | 1 | 1/11 H (hydrogen) | 5 | 5/11 Check: 1/11 + 4/11 + 1/11 + 5/11 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent Sr (strontium) | 1 | 1/11 × 100% = 9.09% O (oxygen) | 4 | 4/11 × 100% = 36.4% Li (lithium) | 1 | 1/11 × 100% = 9.09% H (hydrogen) | 5 | 5/11 × 100% = 45.5% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u Sr (strontium) | 1 | 9.09% | 87.62 O (oxygen) | 4 | 36.4% | 15.999 Li (lithium) | 1 | 9.09% | 6.94 H (hydrogen) | 5 | 45.5% | 1.008 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u Sr (strontium) | 1 | 9.09% | 87.62 | 1 × 87.62 = 87.62 O (oxygen) | 4 | 36.4% | 15.999 | 4 × 15.999 = 63.996 Li (lithium) | 1 | 9.09% | 6.94 | 1 × 6.94 = 6.94 H (hydrogen) | 5 | 45.5% | 1.008 | 5 × 1.008 = 5.040 m = 87.62 u + 63.996 u + 6.94 u + 5.040 u = 163.596 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction Sr (strontium) | 1 | 9.09% | 87.62/163.596 O (oxygen) | 4 | 36.4% | 63.996/163.596 Li (lithium) | 1 | 9.09% | 6.94/163.596 H (hydrogen) | 5 | 45.5% | 5.040/163.596 Check: 87.62/163.596 + 63.996/163.596 + 6.94/163.596 + 5.040/163.596 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent Sr (strontium) | 1 | 9.09% | 87.62/163.596 × 100% = 53.56% O (oxygen) | 4 | 36.4% | 63.996/163.596 × 100% = 39.12% Li (lithium) | 1 | 9.09% | 6.94/163.596 × 100% = 4.24% H (hydrogen) | 5 | 45.5% | 5.040/163.596 × 100% = 3.081%
Elemental oxidation states
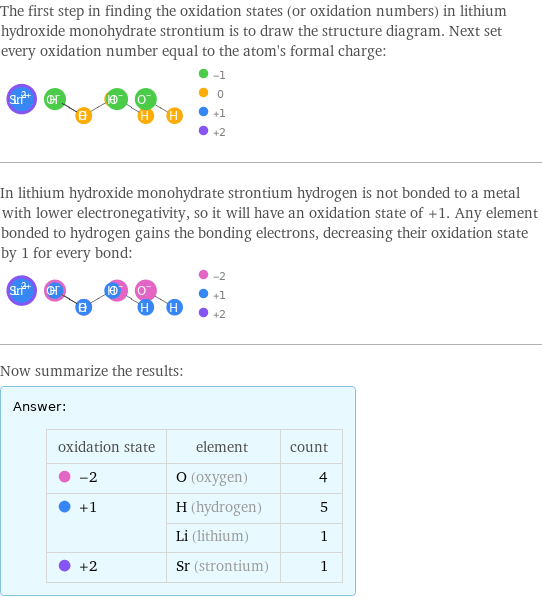
The first step in finding the oxidation states (or oxidation numbers) in lithium hydroxide monohydrate strontium is to draw the structure diagram. Next set every oxidation number equal to the atom's formal charge: In lithium hydroxide monohydrate strontium hydrogen is not bonded to a metal with lower electronegativity, so it will have an oxidation state of +1. Any element bonded to hydrogen gains the bonding electrons, decreasing their oxidation state by 1 for every bond: Now summarize the results: Answer: | | oxidation state | element | count -2 | O (oxygen) | 4 +1 | H (hydrogen) | 5 | Li (lithium) | 1 +2 | Sr (strontium) | 1
Orbital hybridization
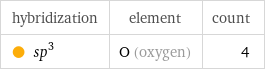
hybridization | element | count sp^3 | O (oxygen) | 4
Structure diagram
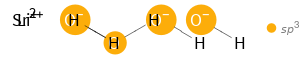
Orbital hybridization Structure diagram
Topological indices
vertex count | 11 edge count | 5 Schultz index | Wiener index | Hosoya index | Balaban index |