Input interpretation

chlorofluorocarbons | molecular mass
Summary
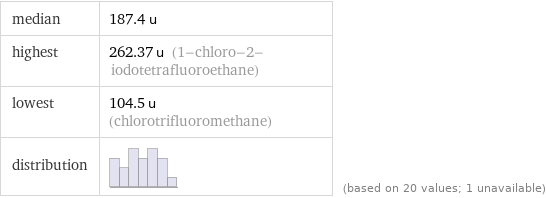
median | 187.4 u highest | 262.37 u (1-chloro-2-iodotetrafluoroethane) lowest | 104.5 u (chlorotrifluoromethane) distribution | | (based on 20 values; 1 unavailable)
Entity with missing value
ethene, chlorotrifluoro-, homopolymer
Distribution plots
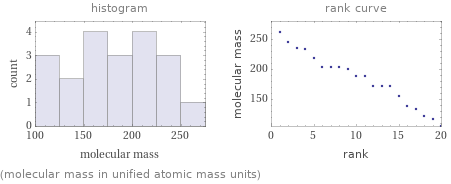
(molecular mass in unified atomic mass units)
Molecular mass rankings
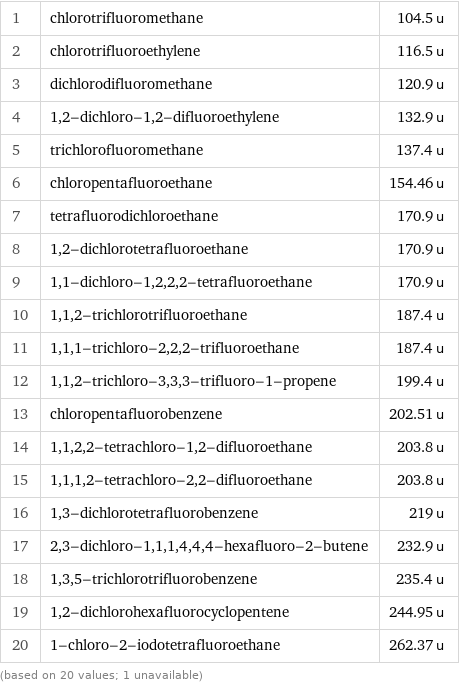
1 | chlorotrifluoromethane | 104.5 u 2 | chlorotrifluoroethylene | 116.5 u 3 | dichlorodifluoromethane | 120.9 u 4 | 1, 2-dichloro-1, 2-difluoroethylene | 132.9 u 5 | trichlorofluoromethane | 137.4 u 6 | chloropentafluoroethane | 154.46 u 7 | tetrafluorodichloroethane | 170.9 u 8 | 1, 2-dichlorotetrafluoroethane | 170.9 u 9 | 1, 1-dichloro-1, 2, 2, 2-tetrafluoroethane | 170.9 u 10 | 1, 1, 2-trichlorotrifluoroethane | 187.4 u 11 | 1, 1, 1-trichloro-2, 2, 2-trifluoroethane | 187.4 u 12 | 1, 1, 2-trichloro-3, 3, 3-trifluoro-1-propene | 199.4 u 13 | chloropentafluorobenzene | 202.51 u 14 | 1, 1, 2, 2-tetrachloro-1, 2-difluoroethane | 203.8 u 15 | 1, 1, 1, 2-tetrachloro-2, 2-difluoroethane | 203.8 u 16 | 1, 3-dichlorotetrafluorobenzene | 219 u 17 | 2, 3-dichloro-1, 1, 1, 4, 4, 4-hexafluoro-2-butene | 232.9 u 18 | 1, 3, 5-trichlorotrifluorobenzene | 235.4 u 19 | 1, 2-dichlorohexafluorocyclopentene | 244.95 u 20 | 1-chloro-2-iodotetrafluoroethane | 262.37 u (based on 20 values; 1 unavailable)
Unit conversions for median molecular mass 187.4 u
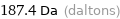
187.4 Da (daltons)
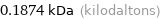
0.1874 kDa (kilodaltons)
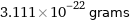
3.111×10^-22 grams
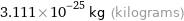
3.111×10^-25 kg (kilograms)
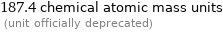
187.4 chemical atomic mass units (unit officially deprecated)
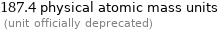
187.4 physical atomic mass units (unit officially deprecated)
Comparisons for median molecular mass 187.4 u
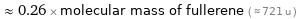
≈ 0.26 × molecular mass of fullerene ( ≈ 721 u )
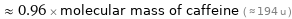
≈ 0.96 × molecular mass of caffeine ( ≈ 194 u )
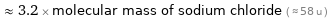
≈ 3.2 × molecular mass of sodium chloride ( ≈ 58 u )
Corresponding quantities
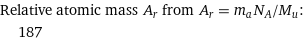
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 187
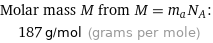
Molar mass M from M = m_aN_A: | 187 g/mol (grams per mole)
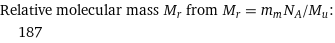
Relative molecular mass M_r from M_r = m_mN_A/M_u: | 187
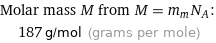
Molar mass M from M = m_mN_A: | 187 g/mol (grams per mole)