Input interpretation
transition metal ions
Members

scandium(III) cation | titanium(IV) cation | vanadium(V) cation | chromium(III) cation | manganese(II) cation | manganese(IV) cation | iron(II) cation | iron(III) cation | cobalt(II) cation | cobalt(III) cation | nickel(II) cation | copper(II) cation | zinc cation | palladium(II) cation | palladium(IV) cation | silver(I) cation | cadmium cation | gold(III) cation | dimercury(I) cation | mercury(II) cation (total: 20)
Ionic radii
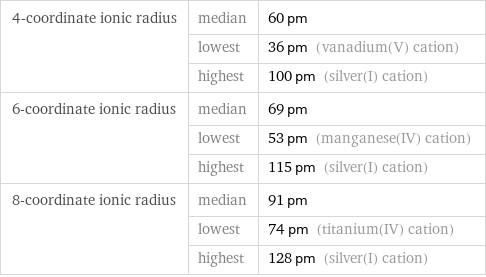
4-coordinate ionic radius | median | 60 pm | lowest | 36 pm (vanadium(V) cation) | highest | 100 pm (silver(I) cation) 6-coordinate ionic radius | median | 69 pm | lowest | 53 pm (manganese(IV) cation) | highest | 115 pm (silver(I) cation) 8-coordinate ionic radius | median | 91 pm | lowest | 74 pm (titanium(IV) cation) | highest | 128 pm (silver(I) cation)
Units
