Input interpretation
mercuric chloride
Chemical names and formulas
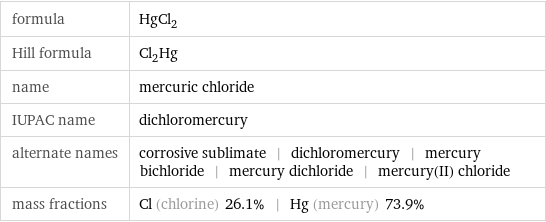
formula | HgCl_2 Hill formula | Cl_2Hg name | mercuric chloride IUPAC name | dichloromercury alternate names | corrosive sublimate | dichloromercury | mercury bichloride | mercury dichloride | mercury(II) chloride mass fractions | Cl (chlorine) 26.1% | Hg (mercury) 73.9%
Structure diagram

Structure diagram

| bond counts | bond lengths | 2 bonds | 2.4 Å
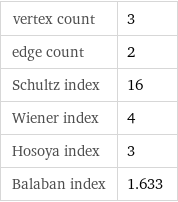
vertex count | 3 edge count | 2 Schultz index | 16 Wiener index | 4 Hosoya index | 3 Balaban index | 1.633
Basic properties
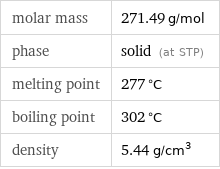
molar mass | 271.49 g/mol phase | solid (at STP) melting point | 277 °C boiling point | 302 °C density | 5.44 g/cm^3
Units

Solid properties (at STP)
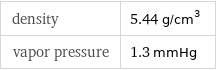
density | 5.44 g/cm^3 vapor pressure | 1.3 mmHg
Units

Thermodynamic properties
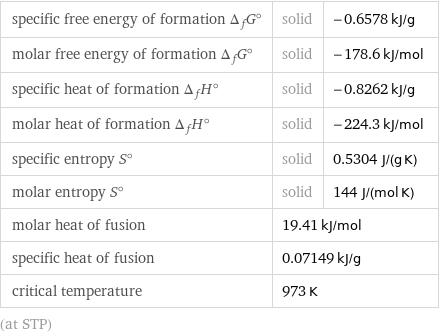
specific free energy of formation Δ_fG° | solid | -0.6578 kJ/g molar free energy of formation Δ_fG° | solid | -178.6 kJ/mol specific heat of formation Δ_fH° | solid | -0.8262 kJ/g molar heat of formation Δ_fH° | solid | -224.3 kJ/mol specific entropy S° | solid | 0.5304 J/(g K) molar entropy S° | solid | 144 J/(mol K) molar heat of fusion | 19.41 kJ/mol | specific heat of fusion | 0.07149 kJ/g | critical temperature | 973 K | (at STP)
Chemical identifiers
![CAS number | 7487-94-7 PubChem CID number | 24085 PubChem SID number | 24852198 SMILES identifier | Cl[Hg]Cl InChI identifier | InChI=1/2ClH.Hg/h2*1H;/q;;+2/p-2/f2Cl.Hg/h2*1h;/q2*-1;m RTECS number | OV9100000 MDL number | MFCD00011041](../image_source/be2ee173243de210ba395fae6fc968b2.png)
CAS number | 7487-94-7 PubChem CID number | 24085 PubChem SID number | 24852198 SMILES identifier | Cl[Hg]Cl InChI identifier | InChI=1/2ClH.Hg/h2*1H;/q;;+2/p-2/f2Cl.Hg/h2*1h;/q2*-1;m RTECS number | OV9100000 MDL number | MFCD00011041
NFPA label

NFPA label

NFPA health rating | 4 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties
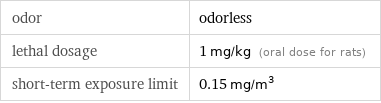
odor | odorless lethal dosage | 1 mg/kg (oral dose for rats) short-term exposure limit | 0.15 mg/m^3

probable lethal dose for man | 1 drop long-term exposure limit | 0.05 mg/m^3 (over 8 hours) RTECS classes | agricultural chemical and pesticide | tumorigen | drug | mutagen | reproductive effector | human data | primary irritant
Ion equivalents

Hg^(2+) (mercury(II) cation) | 1 Cl^- (chloride anion) | 2