Input interpretation

f-block elements | specific heat at STP
Summary
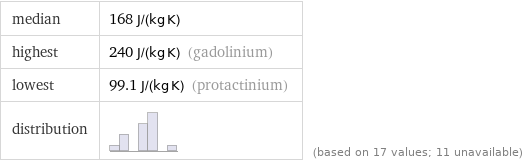
median | 168 J/(kg K) highest | 240 J/(kg K) (gadolinium) lowest | 99.1 J/(kg K) (protactinium) distribution | | (based on 17 values; 11 unavailable)
Entities with missing values

promethium | neptunium | plutonium | americium | curium | berkelium | californium | einsteinium | fermium | mendelevium | nobelium (total: 11)
Distribution plots
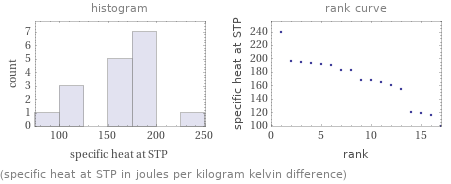
(specific heat at STP in joules per kilogram kelvin difference)
Specific heat at STP rankings
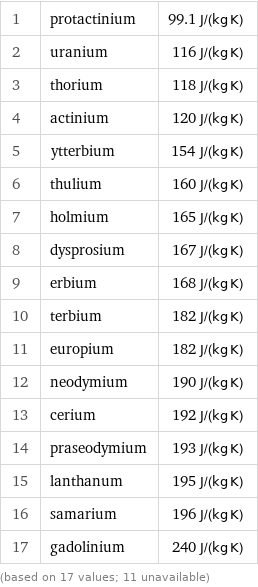
1 | protactinium | 99.1 J/(kg K) 2 | uranium | 116 J/(kg K) 3 | thorium | 118 J/(kg K) 4 | actinium | 120 J/(kg K) 5 | ytterbium | 154 J/(kg K) 6 | thulium | 160 J/(kg K) 7 | holmium | 165 J/(kg K) 8 | dysprosium | 167 J/(kg K) 9 | erbium | 168 J/(kg K) 10 | terbium | 182 J/(kg K) 11 | europium | 182 J/(kg K) 12 | neodymium | 190 J/(kg K) 13 | cerium | 192 J/(kg K) 14 | praseodymium | 193 J/(kg K) 15 | lanthanum | 195 J/(kg K) 16 | samarium | 196 J/(kg K) 17 | gadolinium | 240 J/(kg K) (based on 17 values; 11 unavailable)
Unit conversions for median specific heat at STP 168 J/(kg K)
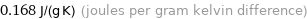
0.168 J/(g K) (joules per gram kelvin difference)
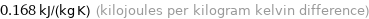
0.168 kJ/(kg K) (kilojoules per kilogram kelvin difference)
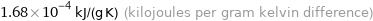
1.68×10^-4 kJ/(g K) (kilojoules per gram kelvin difference)

168 J/(kg °C) (joules per kilogram degree Celsius difference)
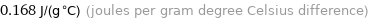
0.168 J/(g °C) (joules per gram degree Celsius difference)

0.0401 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons for median specific heat at STP 168 J/(kg K)
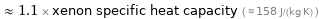
≈ 1.1 × xenon specific heat capacity ( ≈ 158 J/(kg K) )
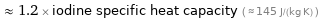
≈ 1.2 × iodine specific heat capacity ( ≈ 145 J/(kg K) )
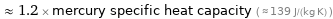
≈ 1.2 × mercury specific heat capacity ( ≈ 139 J/(kg K) )
Thermodynamic properties
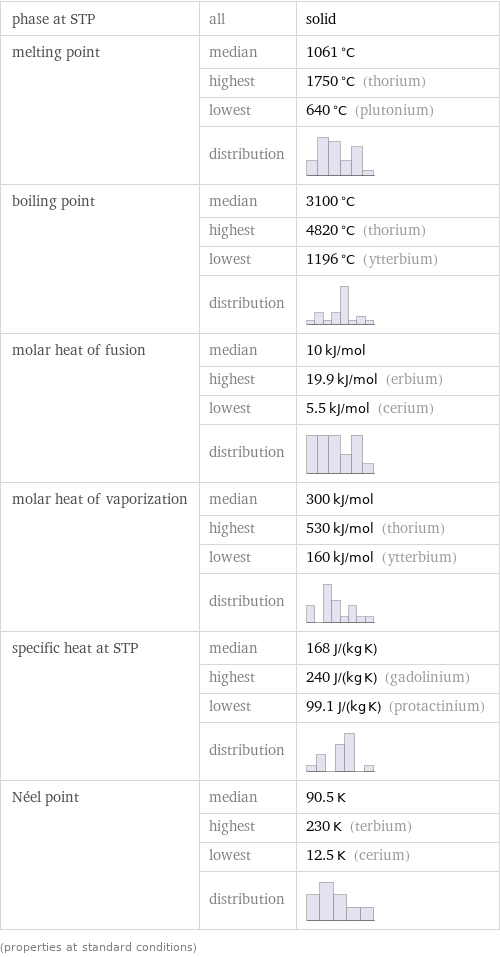
phase at STP | all | solid melting point | median | 1061 °C | highest | 1750 °C (thorium) | lowest | 640 °C (plutonium) | distribution | boiling point | median | 3100 °C | highest | 4820 °C (thorium) | lowest | 1196 °C (ytterbium) | distribution | molar heat of fusion | median | 10 kJ/mol | highest | 19.9 kJ/mol (erbium) | lowest | 5.5 kJ/mol (cerium) | distribution | molar heat of vaporization | median | 300 kJ/mol | highest | 530 kJ/mol (thorium) | lowest | 160 kJ/mol (ytterbium) | distribution | specific heat at STP | median | 168 J/(kg K) | highest | 240 J/(kg K) (gadolinium) | lowest | 99.1 J/(kg K) (protactinium) | distribution | Néel point | median | 90.5 K | highest | 230 K (terbium) | lowest | 12.5 K (cerium) | distribution | (properties at standard conditions)