Input interpretation
lithium carbonate
Chemical names and formulas
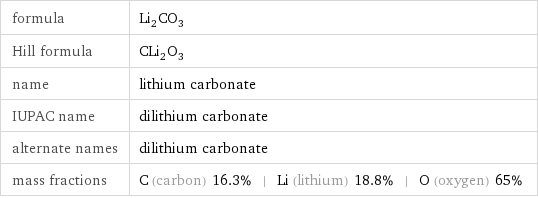
formula | Li_2CO_3 Hill formula | CLi_2O_3 name | lithium carbonate IUPAC name | dilithium carbonate alternate names | dilithium carbonate mass fractions | C (carbon) 16.3% | Li (lithium) 18.8% | O (oxygen) 65%
Structure diagram
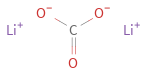
Structure diagram
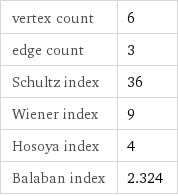
vertex count | 6 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties
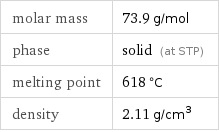
molar mass | 73.9 g/mol phase | solid (at STP) melting point | 618 °C density | 2.11 g/cm^3
Units

Solid properties (at STP)

density | 2.11 g/cm^3
Units

Thermodynamic properties
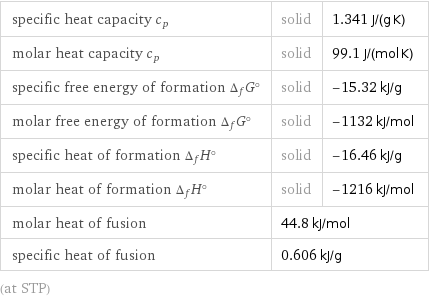
specific heat capacity c_p | solid | 1.341 J/(g K) molar heat capacity c_p | solid | 99.1 J/(mol K) specific free energy of formation Δ_fG° | solid | -15.32 kJ/g molar free energy of formation Δ_fG° | solid | -1132 kJ/mol specific heat of formation Δ_fH° | solid | -16.46 kJ/g molar heat of formation Δ_fH° | solid | -1216 kJ/mol molar heat of fusion | 44.8 kJ/mol | specific heat of fusion | 0.606 kJ/g | (at STP)
Chemical identifiers
![CAS number | 554-13-2 Beilstein number | 3999191 PubChem CID number | 11125 PubChem SID number | 24852186 SMILES identifier | [Li+].[Li+].C(=O)([O-])[O-] InChI identifier | InChI=1/CH2O3.2Li/c2-1(3)4;;/h(H2, 2, 3, 4);;/q;2*+1/p-2/fCO3.2Li/q-2;2m RTECS number | OJ5800000 MDL number | MFCD00011084](../image_source/4e3fba994fe192f4708c38b2231b1cf4.png)
CAS number | 554-13-2 Beilstein number | 3999191 PubChem CID number | 11125 PubChem SID number | 24852186 SMILES identifier | [Li+].[Li+].C(=O)([O-])[O-] InChI identifier | InChI=1/CH2O3.2Li/c2-1(3)4;;/h(H2, 2, 3, 4);;/q;2*+1/p-2/fCO3.2Li/q-2;2m RTECS number | OJ5800000 MDL number | MFCD00011084
Toxicity properties
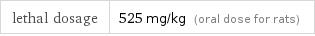
lethal dosage | 525 mg/kg (oral dose for rats)
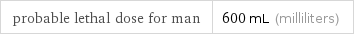
probable lethal dose for man | 600 mL (milliliters)
Ion equivalents

Li^+ (lithium cation) | 2 (CO_3)^(2-) (carbonate anion) | 1