Input interpretation

polyatomic ions | molar mass
Summary
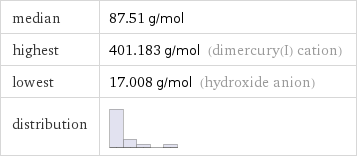
median | 87.51 g/mol highest | 401.183 g/mol (dimercury(I) cation) lowest | 17.008 g/mol (hydroxide anion) distribution |
Units

Distribution plots
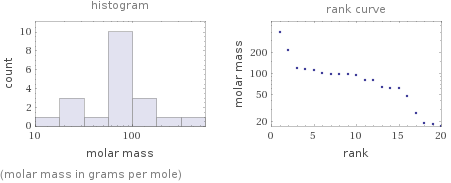
(molar mass in grams per mole)
Molar mass rankings
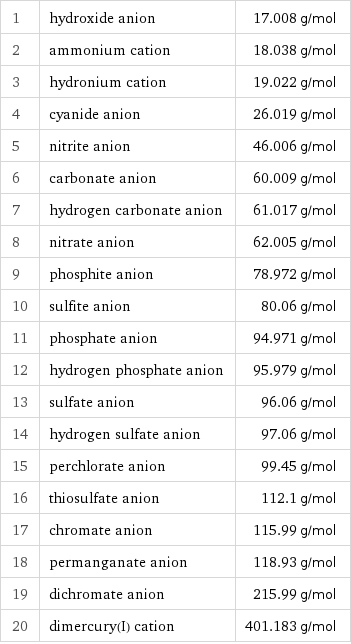
1 | hydroxide anion | 17.008 g/mol 2 | ammonium cation | 18.038 g/mol 3 | hydronium cation | 19.022 g/mol 4 | cyanide anion | 26.019 g/mol 5 | nitrite anion | 46.006 g/mol 6 | carbonate anion | 60.009 g/mol 7 | hydrogen carbonate anion | 61.017 g/mol 8 | nitrate anion | 62.005 g/mol 9 | phosphite anion | 78.972 g/mol 10 | sulfite anion | 80.06 g/mol 11 | phosphate anion | 94.971 g/mol 12 | hydrogen phosphate anion | 95.979 g/mol 13 | sulfate anion | 96.06 g/mol 14 | hydrogen sulfate anion | 97.06 g/mol 15 | perchlorate anion | 99.45 g/mol 16 | thiosulfate anion | 112.1 g/mol 17 | chromate anion | 115.99 g/mol 18 | permanganate anion | 118.93 g/mol 19 | dichromate anion | 215.99 g/mol 20 | dimercury(I) cation | 401.183 g/mol
Unit conversion for median molar mass 87.51 g/mol
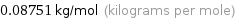
0.08751 kg/mol (kilograms per mole)
Comparisons for median molar mass 87.51 g/mol
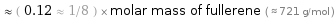
≈ ( 0.12 ≈ 1/8 ) × molar mass of fullerene ( ≈ 721 g/mol )
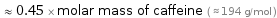
≈ 0.45 × molar mass of caffeine ( ≈ 194 g/mol )
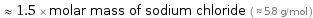
≈ 1.5 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
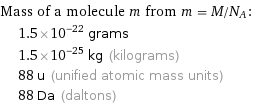
Mass of a molecule m from m = M/N_A: | 1.5×10^-22 grams | 1.5×10^-25 kg (kilograms) | 88 u (unified atomic mass units) | 88 Da (daltons)
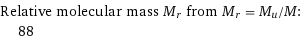
Relative molecular mass M_r from M_r = M_u/M: | 88