Input interpretation
potassium permanganate
Chemical names and formulas

formula | KMnO_4 name | potassium permanganate alternate names | cairox | chameleon mineral | condy's crystals | permanganate of potash | potassium manganate(VII) | potassium permanganate(VII) mass fractions | K (potassium) 24.7% | Mn (manganese) 34.8% | O (oxygen) 40.5%
Structure diagram
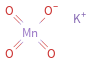
Structure diagram
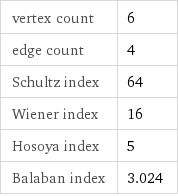
vertex count | 6 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
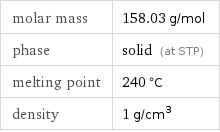
molar mass | 158.03 g/mol phase | solid (at STP) melting point | 240 °C density | 1 g/cm^3
Units

Solid properties (at STP)

density | 1 g/cm^3 vapor pressure | 0.075 mmHg (at 20 °C)
Units

Thermodynamic properties
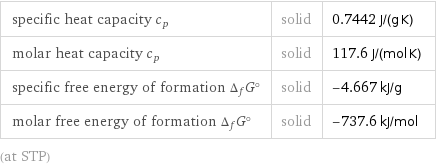
specific heat capacity c_p | solid | 0.7442 J/(g K) molar heat capacity c_p | solid | 117.6 J/(mol K) specific free energy of formation Δ_fG° | solid | -4.667 kJ/g molar free energy of formation Δ_fG° | solid | -737.6 kJ/mol (at STP)
Chemical identifiers
![CAS number | 7722-64-7 PubChem CID number | 516875 PubChem SID number | 24859164 SMILES identifier | [O-][Mn](=O)(=O)=O.[K+] InChI identifier | InChI=1/K.Mn.4O/q+1;;;;;-1/rK.MnO4/c;2-1(3, 4)5/q+1;-1 RTECS number | SD6475000 MDL number | MFCD00011364](../image_source/38ac5f797c8933fcb400bfe1fc0eda74.png)
CAS number | 7722-64-7 PubChem CID number | 516875 PubChem SID number | 24859164 SMILES identifier | [O-][Mn](=O)(=O)=O.[K+] InChI identifier | InChI=1/K.Mn.4O/q+1;;;;;-1/rK.MnO4/c;2-1(3, 4)5/q+1;-1 RTECS number | SD6475000 MDL number | MFCD00011364
NFPA label
NFPA label
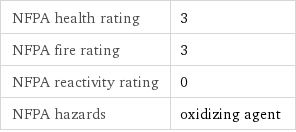
NFPA health rating | 3 NFPA fire rating | 3 NFPA reactivity rating | 0 NFPA hazards | oxidizing agent
Toxicity properties

odor | odorless

RTECS classes | agricultural chemical and pesticide | mutagen | reproductive effector | human data
Ion equivalents

K^+ (potassium cation) | 1 (MnO_4)^- (permanganate anion) | 1