Input interpretation
poor metals | specific heat at STP
Summary

median | 217 J/(kg K) highest | 904 J/(kg K) (aluminum) lowest | 122 J/(kg K) (bismuth) distribution | | (based on 7 values; 4 unavailable)
Entities with missing values
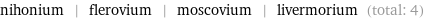
nihonium | flerovium | moscovium | livermorium (total: 4)
Specific heat at STP rankings
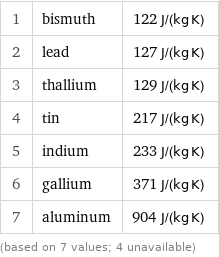
1 | bismuth | 122 J/(kg K) 2 | lead | 127 J/(kg K) 3 | thallium | 129 J/(kg K) 4 | tin | 217 J/(kg K) 5 | indium | 233 J/(kg K) 6 | gallium | 371 J/(kg K) 7 | aluminum | 904 J/(kg K) (based on 7 values; 4 unavailable)
Unit conversions for median specific heat at STP 217 J/(kg K)
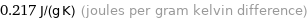
0.217 J/(g K) (joules per gram kelvin difference)
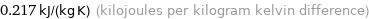
0.217 kJ/(kg K) (kilojoules per kilogram kelvin difference)
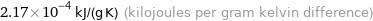
2.17×10^-4 kJ/(g K) (kilojoules per gram kelvin difference)

217 J/(kg °C) (joules per kilogram degree Celsius difference)
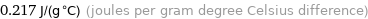
0.217 J/(g °C) (joules per gram degree Celsius difference)

0.0518 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons for median specific heat at STP 217 J/(kg K)
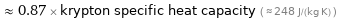
≈ 0.87 × krypton specific heat capacity ( ≈ 248 J/(kg K) )
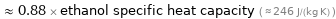
≈ 0.88 × ethanol specific heat capacity ( ≈ 246 J/(kg K) )
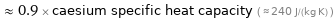
≈ 0.9 × caesium specific heat capacity ( ≈ 240 J/(kg K) )
Thermodynamic properties
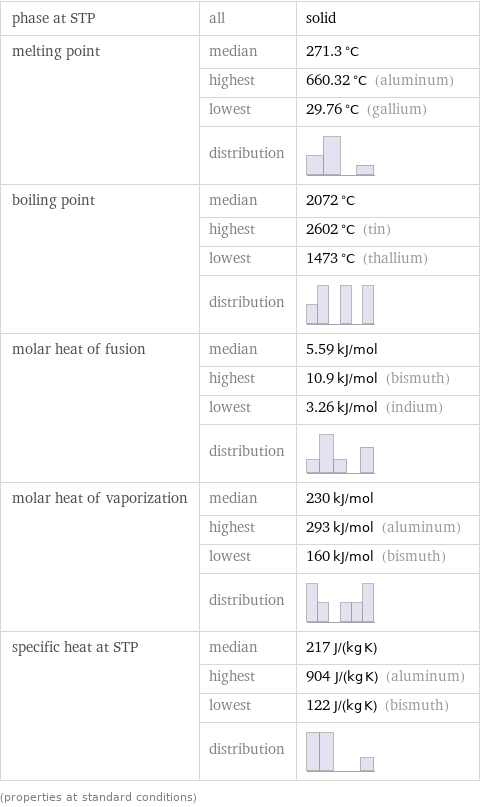
phase at STP | all | solid melting point | median | 271.3 °C | highest | 660.32 °C (aluminum) | lowest | 29.76 °C (gallium) | distribution | boiling point | median | 2072 °C | highest | 2602 °C (tin) | lowest | 1473 °C (thallium) | distribution | molar heat of fusion | median | 5.59 kJ/mol | highest | 10.9 kJ/mol (bismuth) | lowest | 3.26 kJ/mol (indium) | distribution | molar heat of vaporization | median | 230 kJ/mol | highest | 293 kJ/mol (aluminum) | lowest | 160 kJ/mol (bismuth) | distribution | specific heat at STP | median | 217 J/(kg K) | highest | 904 J/(kg K) (aluminum) | lowest | 122 J/(kg K) (bismuth) | distribution | (properties at standard conditions)