Input interpretation
stibine | nickel(II) stannate dihydrate
Chemical names and formulas
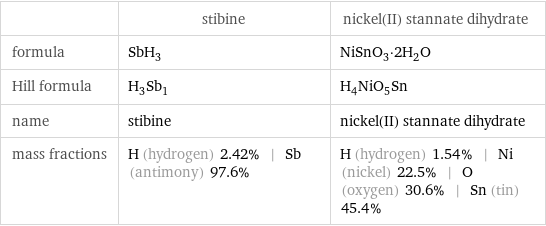
| stibine | nickel(II) stannate dihydrate formula | SbH_3 | NiSnO_3·2H_2O Hill formula | H_3Sb_1 | H_4NiO_5Sn name | stibine | nickel(II) stannate dihydrate mass fractions | H (hydrogen) 2.42% | Sb (antimony) 97.6% | H (hydrogen) 1.54% | Ni (nickel) 22.5% | O (oxygen) 30.6% | Sn (tin) 45.4%
Structure diagrams
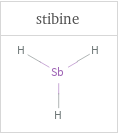
Structure diagrams
Basic properties
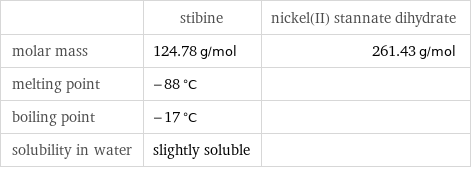
| stibine | nickel(II) stannate dihydrate molar mass | 124.78 g/mol | 261.43 g/mol melting point | -88 °C | boiling point | -17 °C | solubility in water | slightly soluble |
Units
