Input interpretation
copper(II) sulfate
Chemical names and formulas

formula | CuSO_4 Hill formula | CuO_4S name | copper(II) sulfate IUPAC name | copper sulfate alternate names | cupric sulfate mass fractions | Cu (copper) 39.8% | O (oxygen) 40.1% | S (sulfur) 20.1%
Structure diagram
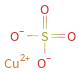
Structure diagram
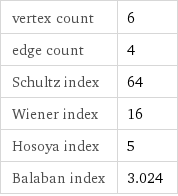
vertex count | 6 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
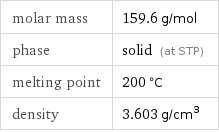
molar mass | 159.6 g/mol phase | solid (at STP) melting point | 200 °C density | 3.603 g/cm^3
Units

Solid properties (at STP)

density | 3.603 g/cm^3 vapor pressure | 7.299 mmHg
Units

Thermodynamic properties
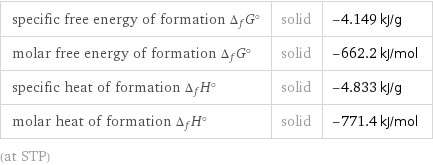
specific free energy of formation Δ_fG° | solid | -4.149 kJ/g molar free energy of formation Δ_fG° | solid | -662.2 kJ/mol specific heat of formation Δ_fH° | solid | -4.833 kJ/g molar heat of formation Δ_fH° | solid | -771.4 kJ/mol (at STP)
Chemical identifiers
![CAS number | 7758-98-7 PubChem CID number | 24462 SMILES identifier | [O-]S(=O)(=O)[O-].[Cu+2] InChI identifier | InChI=1/Cu.H2O4S/c;1-5(2, 3)4/h;(H2, 1, 2, 3, 4)/q+2;/p-2/fCu.O4S/qm;-2 RTECS number | GL8800000 MDL number | MFCD00010981](../image_source/fac8d9706b8afda3dd5a3a44da0275eb.png)
CAS number | 7758-98-7 PubChem CID number | 24462 SMILES identifier | [O-]S(=O)(=O)[O-].[Cu+2] InChI identifier | InChI=1/Cu.H2O4S/c;1-5(2, 3)4/h;(H2, 1, 2, 3, 4)/q+2;/p-2/fCu.O4S/qm;-2 RTECS number | GL8800000 MDL number | MFCD00010981
Toxicity properties

lethal dosage | 300 mg/kg (oral dose for rats) short-term exposure limit | 2 mg/m^3

probable lethal dose for man | 30 mL (milliliters) long-term exposure limit | 1 mg/m^3 (over 8 hours)
Ion equivalents

Cu^(2+) (copper(II) cation) | 1 (SO_4)^(2-) (sulfate anion) | 1