Input interpretation
biomolecule ions
Members

alkyl halide | D-threo-aldose | aluminum cation | ammonium cation | antimonite anion | arsenic(V) cation | barium cation | beryllium cation | borate anion | borohydride anion | bromide anion | cadmium cation | calcium cation | cesium cation | chlorate anion | chloride anion | chlorite anion | chromate anion | chromium(II) cation | chromium(III) cation | chromium(VI) cation | cobalt(I) cation | cobalt(II) cation | cobalt(III) cation | copper(I) cation | copper(II) cation | electron anion | fluoride anion | hydrogen cation | hydrogen carbonate anion | hydrogen phosphite anion | hydrogen sulfate anion | hydrogen sulfite anion | hydronium cation | hydrosulfide anion | hydroxide anion | iodide anion | iron(II) cation | iron(III) cation | lead(II) cation | ... (total: 68)
Ionic radii
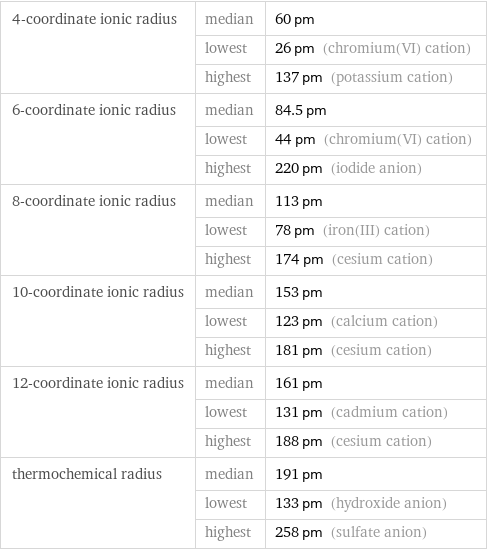
4-coordinate ionic radius | median | 60 pm | lowest | 26 pm (chromium(VI) cation) | highest | 137 pm (potassium cation) 6-coordinate ionic radius | median | 84.5 pm | lowest | 44 pm (chromium(VI) cation) | highest | 220 pm (iodide anion) 8-coordinate ionic radius | median | 113 pm | lowest | 78 pm (iron(III) cation) | highest | 174 pm (cesium cation) 10-coordinate ionic radius | median | 153 pm | lowest | 123 pm (calcium cation) | highest | 181 pm (cesium cation) 12-coordinate ionic radius | median | 161 pm | lowest | 131 pm (cadmium cation) | highest | 188 pm (cesium cation) thermochemical radius | median | 191 pm | lowest | 133 pm (hydroxide anion) | highest | 258 pm (sulfate anion)
Units
