Input interpretation
sodium nitrate
Chemical names and formulas
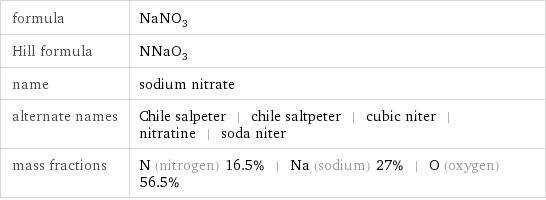
formula | NaNO_3 Hill formula | NNaO_3 name | sodium nitrate alternate names | Chile salpeter | chile saltpeter | cubic niter | nitratine | soda niter mass fractions | N (nitrogen) 16.5% | Na (sodium) 27% | O (oxygen) 56.5%
Structure diagram
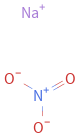
Structure diagram
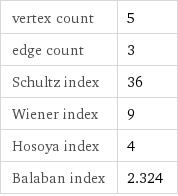
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties
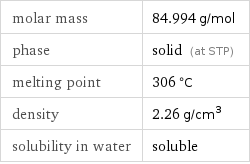
molar mass | 84.994 g/mol phase | solid (at STP) melting point | 306 °C density | 2.26 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)
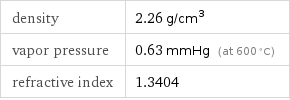
density | 2.26 g/cm^3 vapor pressure | 0.63 mmHg (at 600 °C) refractive index | 1.3404
Units

Thermodynamic properties
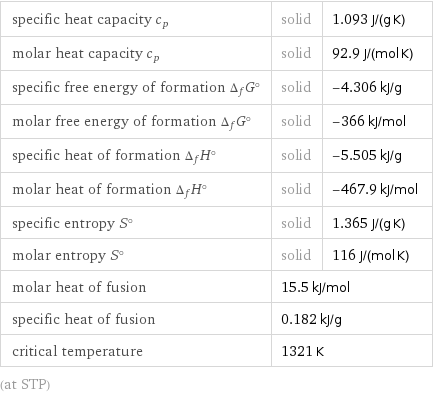
specific heat capacity c_p | solid | 1.093 J/(g K) molar heat capacity c_p | solid | 92.9 J/(mol K) specific free energy of formation Δ_fG° | solid | -4.306 kJ/g molar free energy of formation Δ_fG° | solid | -366 kJ/mol specific heat of formation Δ_fH° | solid | -5.505 kJ/g molar heat of formation Δ_fH° | solid | -467.9 kJ/mol specific entropy S° | solid | 1.365 J/(g K) molar entropy S° | solid | 116 J/(mol K) molar heat of fusion | 15.5 kJ/mol | specific heat of fusion | 0.182 kJ/g | critical temperature | 1321 K | (at STP)
Chemical identifiers
([O-])[O-].[Na+] InChI identifier | InChI=1/NO3.Na/c2-1(3)4;/q-1;+1 RTECS number | WC5600000 MDL number | MFCD00011119](../image_source/08fa8f8c23d8dbbec6d02a3a5db5437b.png)
CAS number | 7631-99-4 PubChem CID number | 24268 PubChem SID number | 24853760 SMILES identifier | [N+](=O)([O-])[O-].[Na+] InChI identifier | InChI=1/NO3.Na/c2-1(3)4;/q-1;+1 RTECS number | WC5600000 MDL number | MFCD00011119
NFPA label
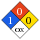
NFPA label
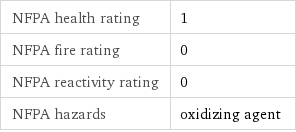
NFPA health rating | 1 NFPA fire rating | 0 NFPA reactivity rating | 0 NFPA hazards | oxidizing agent
Toxicity properties

lethal dosage | 3236 mg/kg (oral dose for rats)

probable lethal dose for man | 600 mL (milliliters) RTECS classes | tumorigen | mutagen | reproductive effector | human data
Ion equivalents

Na^+ (sodium cation) | 1 (NO_3)^- (nitrate anion) | 1