Input interpretation
lithium chloride | rubidium chloride
Chemical names and formulas
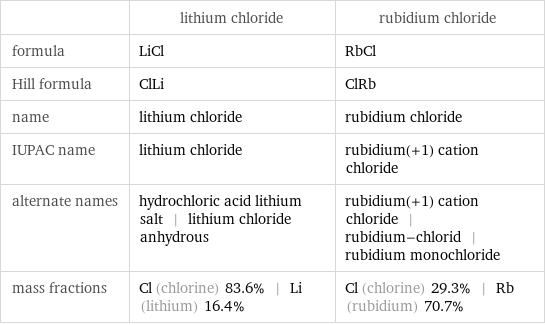
| lithium chloride | rubidium chloride formula | LiCl | RbCl Hill formula | ClLi | ClRb name | lithium chloride | rubidium chloride IUPAC name | lithium chloride | rubidium(+1) cation chloride alternate names | hydrochloric acid lithium salt | lithium chloride anhydrous | rubidium(+1) cation chloride | rubidium-chlorid | rubidium monochloride mass fractions | Cl (chlorine) 83.6% | Li (lithium) 16.4% | Cl (chlorine) 29.3% | Rb (rubidium) 70.7%
Structure diagrams

Structure diagrams
Basic properties
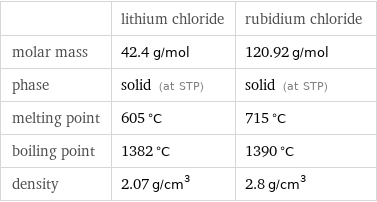
| lithium chloride | rubidium chloride molar mass | 42.4 g/mol | 120.92 g/mol phase | solid (at STP) | solid (at STP) melting point | 605 °C | 715 °C boiling point | 1382 °C | 1390 °C density | 2.07 g/cm^3 | 2.8 g/cm^3
Units

Thermodynamic properties
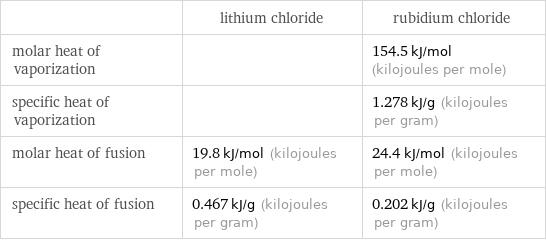
| lithium chloride | rubidium chloride molar heat of vaporization | | 154.5 kJ/mol (kilojoules per mole) specific heat of vaporization | | 1.278 kJ/g (kilojoules per gram) molar heat of fusion | 19.8 kJ/mol (kilojoules per mole) | 24.4 kJ/mol (kilojoules per mole) specific heat of fusion | 0.467 kJ/g (kilojoules per gram) | 0.202 kJ/g (kilojoules per gram)
Solid properties (at STP)
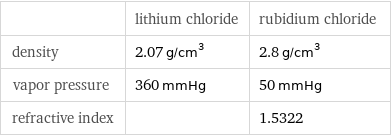
| lithium chloride | rubidium chloride density | 2.07 g/cm^3 | 2.8 g/cm^3 vapor pressure | 360 mmHg | 50 mmHg refractive index | | 1.5322
Units
