Input interpretation
nickel(II) fluoborate | sodium chloride
Chemical names and formulas
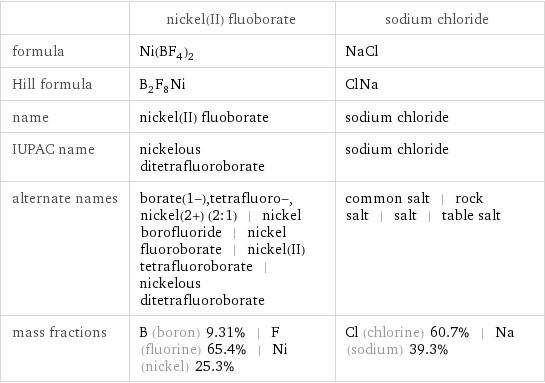
| nickel(II) fluoborate | sodium chloride formula | Ni(BF_4)_2 | NaCl Hill formula | B_2F_8Ni | ClNa name | nickel(II) fluoborate | sodium chloride IUPAC name | nickelous ditetrafluoroborate | sodium chloride alternate names | borate(1-), tetrafluoro-, nickel(2+) (2:1) | nickel borofluoride | nickel fluoroborate | nickel(II) tetrafluoroborate | nickelous ditetrafluoroborate | common salt | rock salt | salt | table salt mass fractions | B (boron) 9.31% | F (fluorine) 65.4% | Ni (nickel) 25.3% | Cl (chlorine) 60.7% | Na (sodium) 39.3%
Structure diagrams
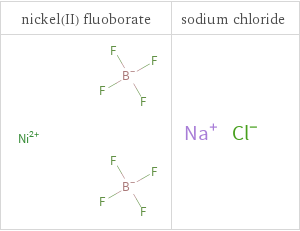
Structure diagrams
Basic properties
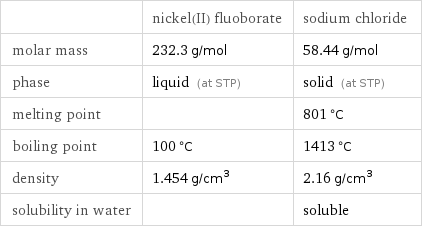
| nickel(II) fluoborate | sodium chloride molar mass | 232.3 g/mol | 58.44 g/mol phase | liquid (at STP) | solid (at STP) melting point | | 801 °C boiling point | 100 °C | 1413 °C density | 1.454 g/cm^3 | 2.16 g/cm^3 solubility in water | | soluble
Units
