Input interpretation

lead(II) hypophosphite | elemental composition
Result
Find the elemental composition for lead(II) hypophosphite in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: Pb(H_2PO_2)_2 Use the chemical formula, Pb(H_2PO_2)_2, to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms: | number of atoms H (hydrogen) | 4 O (oxygen) | 4 P (phosphorus) | 2 Pb (lead) | 1 N_atoms = 4 + 4 + 2 + 1 = 11 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction H (hydrogen) | 4 | 4/11 O (oxygen) | 4 | 4/11 P (phosphorus) | 2 | 2/11 Pb (lead) | 1 | 1/11 Check: 4/11 + 4/11 + 2/11 + 1/11 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent H (hydrogen) | 4 | 4/11 × 100% = 36.4% O (oxygen) | 4 | 4/11 × 100% = 36.4% P (phosphorus) | 2 | 2/11 × 100% = 18.2% Pb (lead) | 1 | 1/11 × 100% = 9.09% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u H (hydrogen) | 4 | 36.4% | 1.008 O (oxygen) | 4 | 36.4% | 15.999 P (phosphorus) | 2 | 18.2% | 30.973761998 Pb (lead) | 1 | 9.09% | 207.2 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u H (hydrogen) | 4 | 36.4% | 1.008 | 4 × 1.008 = 4.032 O (oxygen) | 4 | 36.4% | 15.999 | 4 × 15.999 = 63.996 P (phosphorus) | 2 | 18.2% | 30.973761998 | 2 × 30.973761998 = 61.947523996 Pb (lead) | 1 | 9.09% | 207.2 | 1 × 207.2 = 207.2 m = 4.032 u + 63.996 u + 61.947523996 u + 207.2 u = 337.175523996 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction H (hydrogen) | 4 | 36.4% | 4.032/337.175523996 O (oxygen) | 4 | 36.4% | 63.996/337.175523996 P (phosphorus) | 2 | 18.2% | 61.947523996/337.175523996 Pb (lead) | 1 | 9.09% | 207.2/337.175523996 Check: 4.032/337.175523996 + 63.996/337.175523996 + 61.947523996/337.175523996 + 207.2/337.175523996 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent H (hydrogen) | 4 | 36.4% | 4.032/337.175523996 × 100% = 1.196% O (oxygen) | 4 | 36.4% | 63.996/337.175523996 × 100% = 18.98% P (phosphorus) | 2 | 18.2% | 61.947523996/337.175523996 × 100% = 18.37% Pb (lead) | 1 | 9.09% | 207.2/337.175523996 × 100% = 61.45%
Mass fraction pie chart
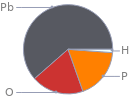
Mass fraction pie chart