Input interpretation
thiophosphoryl chloride | titanium(IV) iodide
Chemical names and formulas
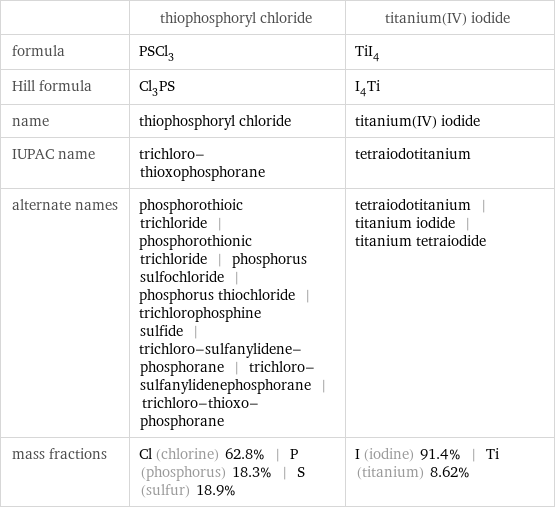
| thiophosphoryl chloride | titanium(IV) iodide formula | PSCl_3 | TiI_4 Hill formula | Cl_3PS | I_4Ti name | thiophosphoryl chloride | titanium(IV) iodide IUPAC name | trichloro-thioxophosphorane | tetraiodotitanium alternate names | phosphorothioic trichloride | phosphorothionic trichloride | phosphorus sulfochloride | phosphorus thiochloride | trichlorophosphine sulfide | trichloro-sulfanylidene-phosphorane | trichloro-sulfanylidenephosphorane | trichloro-thioxo-phosphorane | tetraiodotitanium | titanium iodide | titanium tetraiodide mass fractions | Cl (chlorine) 62.8% | P (phosphorus) 18.3% | S (sulfur) 18.9% | I (iodine) 91.4% | Ti (titanium) 8.62%
Structure diagrams
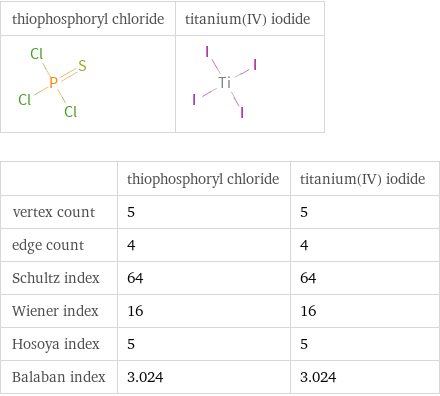
| thiophosphoryl chloride | titanium(IV) iodide vertex count | 5 | 5 edge count | 4 | 4 Schultz index | 64 | 64 Wiener index | 16 | 16 Hosoya index | 5 | 5 Balaban index | 3.024 | 3.024
3D structure

3D structure
Basic properties
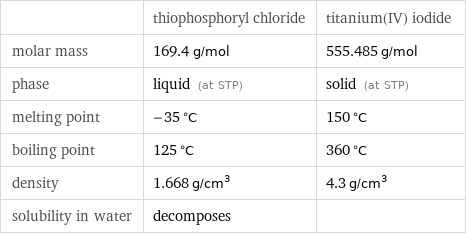
| thiophosphoryl chloride | titanium(IV) iodide molar mass | 169.4 g/mol | 555.485 g/mol phase | liquid (at STP) | solid (at STP) melting point | -35 °C | 150 °C boiling point | 125 °C | 360 °C density | 1.668 g/cm^3 | 4.3 g/cm^3 solubility in water | decomposes |
Units

Liquid properties
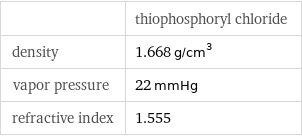
| thiophosphoryl chloride density | 1.668 g/cm^3 vapor pressure | 22 mmHg refractive index | 1.555
Units

Thermodynamic properties
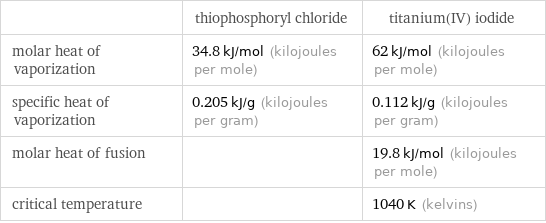
| thiophosphoryl chloride | titanium(IV) iodide molar heat of vaporization | 34.8 kJ/mol (kilojoules per mole) | 62 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.205 kJ/g (kilojoules per gram) | 0.112 kJ/g (kilojoules per gram) molar heat of fusion | | 19.8 kJ/mol (kilojoules per mole) critical temperature | | 1040 K (kelvins)
Solid properties

| titanium(IV) iodide density | 4.3 g/cm^3
Units
