Input interpretation

gray antimony
Chemical names and formulas

formula | Sb name | gray antimony IUPAC name | antimony alternate names | antimony gray | antimony regulus | beta-antimony | crystalline antimony | metallic antimony | rhombohedral antimony | silver antimony mass fractions | Sb (antimony) 100%
Structure diagram

Structure diagram
Basic properties
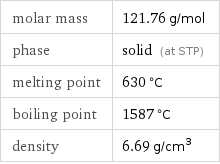
molar mass | 121.76 g/mol phase | solid (at STP) melting point | 630 °C boiling point | 1587 °C density | 6.69 g/cm^3
Units

Solid properties (at STP)

density | 6.69 g/cm^3
Units

Thermodynamic properties
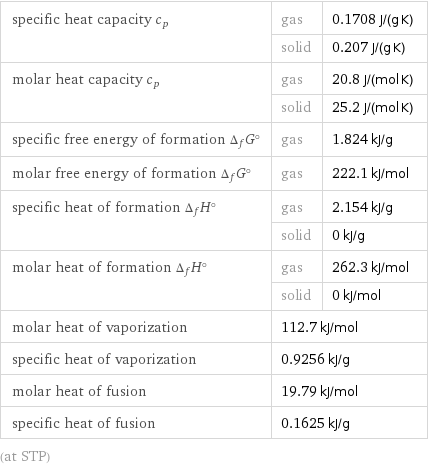
specific heat capacity c_p | gas | 0.1708 J/(g K) | solid | 0.207 J/(g K) molar heat capacity c_p | gas | 20.8 J/(mol K) | solid | 25.2 J/(mol K) specific free energy of formation Δ_fG° | gas | 1.824 kJ/g molar free energy of formation Δ_fG° | gas | 222.1 kJ/mol specific heat of formation Δ_fH° | gas | 2.154 kJ/g | solid | 0 kJ/g molar heat of formation Δ_fH° | gas | 262.3 kJ/mol | solid | 0 kJ/mol molar heat of vaporization | 112.7 kJ/mol | specific heat of vaporization | 0.9256 kJ/g | molar heat of fusion | 19.79 kJ/mol | specific heat of fusion | 0.1625 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7440-36-0 PubChem CID number | 5354495 PubChem SID number | 24852106 SMILES identifier | [Sb] InChI identifier | InChI=1/Sb RTECS number | CC4025000 MDL number | MFCD00134030](../image_source/c8714a77b7c68e72d39cb190a8beb42a.png)
CAS number | 7440-36-0 PubChem CID number | 5354495 PubChem SID number | 24852106 SMILES identifier | [Sb] InChI identifier | InChI=1/Sb RTECS number | CC4025000 MDL number | MFCD00134030