Input interpretation

period 3 elements | phase at STP
Summary
gas | solid
Categorized list

gas | argon, chlorine solid | aluminum, magnesium, phosphorus, silicon, sodium, sulfur
Thermodynamic properties
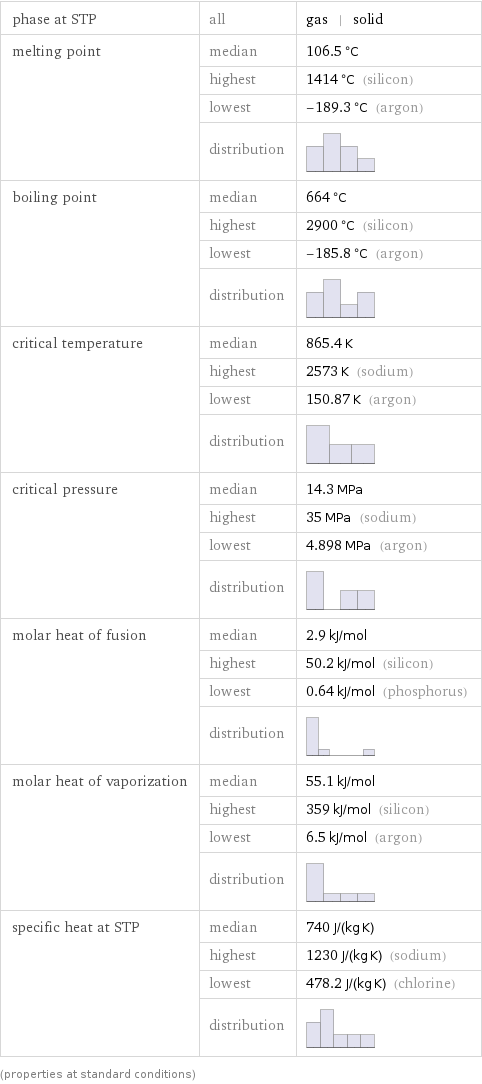
phase at STP | all | gas | solid melting point | median | 106.5 °C | highest | 1414 °C (silicon) | lowest | -189.3 °C (argon) | distribution | boiling point | median | 664 °C | highest | 2900 °C (silicon) | lowest | -185.8 °C (argon) | distribution | critical temperature | median | 865.4 K | highest | 2573 K (sodium) | lowest | 150.87 K (argon) | distribution | critical pressure | median | 14.3 MPa | highest | 35 MPa (sodium) | lowest | 4.898 MPa (argon) | distribution | molar heat of fusion | median | 2.9 kJ/mol | highest | 50.2 kJ/mol (silicon) | lowest | 0.64 kJ/mol (phosphorus) | distribution | molar heat of vaporization | median | 55.1 kJ/mol | highest | 359 kJ/mol (silicon) | lowest | 6.5 kJ/mol (argon) | distribution | specific heat at STP | median | 740 J/(kg K) | highest | 1230 J/(kg K) (sodium) | lowest | 478.2 J/(kg K) (chlorine) | distribution | (properties at standard conditions)