Input interpretation

osmide(2-) anion | ion class
Result
anions | d block ions | group 8 ions | transition metal ions
Ionic radii
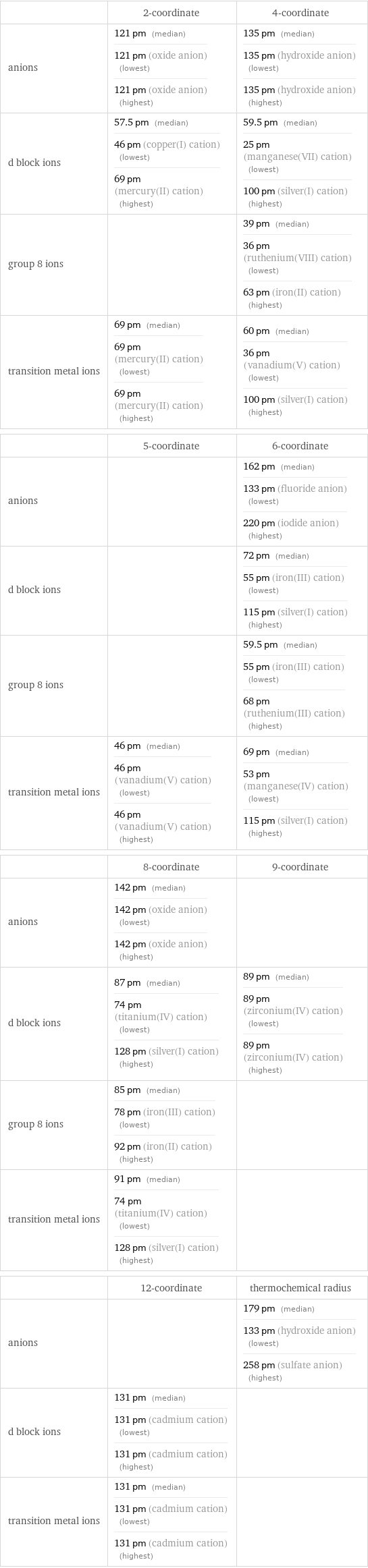
| 2-coordinate | 4-coordinate anions | 121 pm (median) 121 pm (oxide anion) (lowest) 121 pm (oxide anion) (highest) | 135 pm (median) 135 pm (hydroxide anion) (lowest) 135 pm (hydroxide anion) (highest) d block ions | 57.5 pm (median) 46 pm (copper(I) cation) (lowest) 69 pm (mercury(II) cation) (highest) | 59.5 pm (median) 25 pm (manganese(VII) cation) (lowest) 100 pm (silver(I) cation) (highest) group 8 ions | | 39 pm (median) 36 pm (ruthenium(VIII) cation) (lowest) 63 pm (iron(II) cation) (highest) transition metal ions | 69 pm (median) 69 pm (mercury(II) cation) (lowest) 69 pm (mercury(II) cation) (highest) | 60 pm (median) 36 pm (vanadium(V) cation) (lowest) 100 pm (silver(I) cation) (highest) | 5-coordinate | 6-coordinate anions | | 162 pm (median) 133 pm (fluoride anion) (lowest) 220 pm (iodide anion) (highest) d block ions | | 72 pm (median) 55 pm (iron(III) cation) (lowest) 115 pm (silver(I) cation) (highest) group 8 ions | | 59.5 pm (median) 55 pm (iron(III) cation) (lowest) 68 pm (ruthenium(III) cation) (highest) transition metal ions | 46 pm (median) 46 pm (vanadium(V) cation) (lowest) 46 pm (vanadium(V) cation) (highest) | 69 pm (median) 53 pm (manganese(IV) cation) (lowest) 115 pm (silver(I) cation) (highest) | 8-coordinate | 9-coordinate anions | 142 pm (median) 142 pm (oxide anion) (lowest) 142 pm (oxide anion) (highest) | d block ions | 87 pm (median) 74 pm (titanium(IV) cation) (lowest) 128 pm (silver(I) cation) (highest) | 89 pm (median) 89 pm (zirconium(IV) cation) (lowest) 89 pm (zirconium(IV) cation) (highest) group 8 ions | 85 pm (median) 78 pm (iron(III) cation) (lowest) 92 pm (iron(II) cation) (highest) | transition metal ions | 91 pm (median) 74 pm (titanium(IV) cation) (lowest) 128 pm (silver(I) cation) (highest) | | 12-coordinate | thermochemical radius anions | | 179 pm (median) 133 pm (hydroxide anion) (lowest) 258 pm (sulfate anion) (highest) d block ions | 131 pm (median) 131 pm (cadmium cation) (lowest) 131 pm (cadmium cation) (highest) | transition metal ions | 131 pm (median) 131 pm (cadmium cation) (lowest) 131 pm (cadmium cation) (highest) |