Input interpretation
top 10 chemicals | by compressibility factor
Result
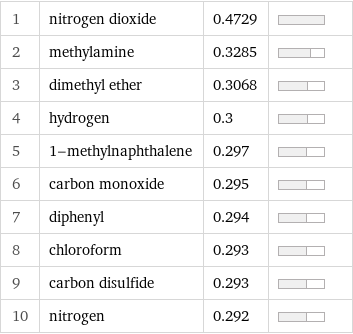
1 | nitrogen dioxide | 0.4729 | 2 | methylamine | 0.3285 | 3 | dimethyl ether | 0.3068 | 4 | hydrogen | 0.3 | 5 | 1-methylnaphthalene | 0.297 | 6 | carbon monoxide | 0.295 | 7 | diphenyl | 0.294 | 8 | chloroform | 0.293 | 9 | carbon disulfide | 0.293 | 10 | nitrogen | 0.292 |
Structure diagrams
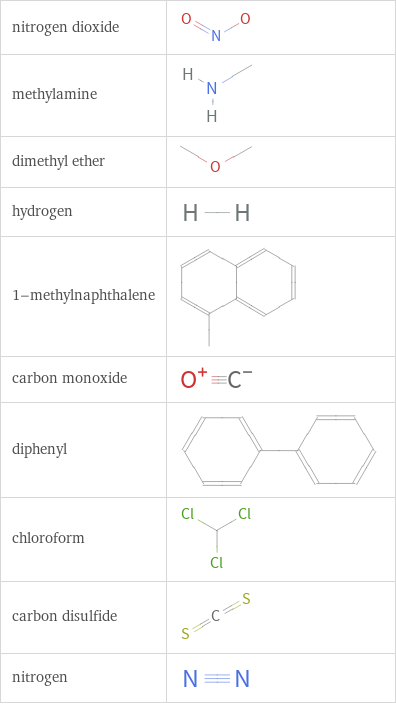
Structure diagrams
Basic properties
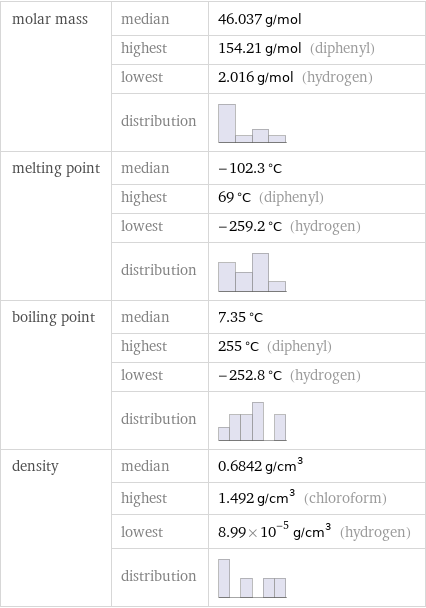
molar mass | median | 46.037 g/mol | highest | 154.21 g/mol (diphenyl) | lowest | 2.016 g/mol (hydrogen) | distribution | melting point | median | -102.3 °C | highest | 69 °C (diphenyl) | lowest | -259.2 °C (hydrogen) | distribution | boiling point | median | 7.35 °C | highest | 255 °C (diphenyl) | lowest | -252.8 °C (hydrogen) | distribution | density | median | 0.6842 g/cm^3 | highest | 1.492 g/cm^3 (chloroform) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution |
Units
