Input interpretation

homonuclear diatomic molecules | boiling point
Summary
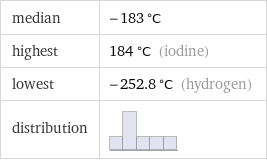
median | -183 °C highest | 184 °C (iodine) lowest | -252.8 °C (hydrogen) distribution |
Boiling point rankings
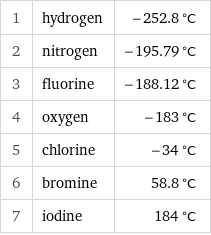
1 | hydrogen | -252.8 °C 2 | nitrogen | -195.79 °C 3 | fluorine | -188.12 °C 4 | oxygen | -183 °C 5 | chlorine | -34 °C 6 | bromine | 58.8 °C 7 | iodine | 184 °C
Unit conversions for median boiling point -183 °C
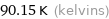
90.15 K (kelvins)

-297.4 °F (degrees Fahrenheit)
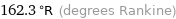
162.3 °R (degrees Rankine)
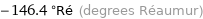
-146.4 °Ré (degrees Réaumur)

-88.58 °Rø (degrees Rømer)
Comparison for median boiling point -183 °C
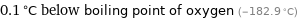
0.1 °C below boiling point of oxygen (-182.9 °C)

12.79 °C above boiling point of nitrogen (-195.79 °C)
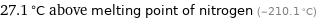
27.1 °C above melting point of nitrogen (-210.1 °C)
Corresponding quantities

Thermodynamic energy E from E = kT: | 7.8 meV (millielectronvolts)
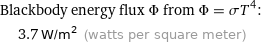
Blackbody energy flux Φ from Φ = σT^4: | 3.7 W/m^2 (watts per square meter)