Input interpretation

organic solvents | molar heat of fusion
Summary
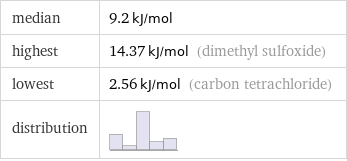
median | 9.2 kJ/mol highest | 14.37 kJ/mol (dimethyl sulfoxide) lowest | 2.56 kJ/mol (carbon tetrachloride) distribution |
Units

Distribution plots
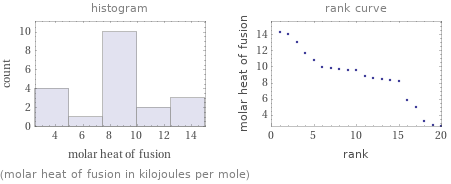
(molar heat of fusion in kilojoules per mole)
Molar heat of fusion rankings
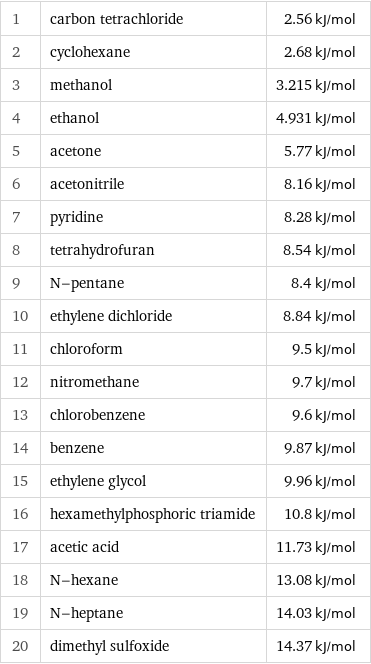
1 | carbon tetrachloride | 2.56 kJ/mol 2 | cyclohexane | 2.68 kJ/mol 3 | methanol | 3.215 kJ/mol 4 | ethanol | 4.931 kJ/mol 5 | acetone | 5.77 kJ/mol 6 | acetonitrile | 8.16 kJ/mol 7 | pyridine | 8.28 kJ/mol 8 | tetrahydrofuran | 8.54 kJ/mol 9 | N-pentane | 8.4 kJ/mol 10 | ethylene dichloride | 8.84 kJ/mol 11 | chloroform | 9.5 kJ/mol 12 | nitromethane | 9.7 kJ/mol 13 | chlorobenzene | 9.6 kJ/mol 14 | benzene | 9.87 kJ/mol 15 | ethylene glycol | 9.96 kJ/mol 16 | hexamethylphosphoric triamide | 10.8 kJ/mol 17 | acetic acid | 11.73 kJ/mol 18 | N-hexane | 13.08 kJ/mol 19 | N-heptane | 14.03 kJ/mol 20 | dimethyl sulfoxide | 14.37 kJ/mol
Unit conversions for median molar heat of fusion 9.2 kJ/mol
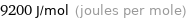
9200 J/mol (joules per mole)
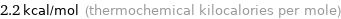
2.2 kcal/mol (thermochemical kilocalories per mole)
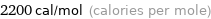
2200 cal/mol (calories per mole)

0.096 eV (molar electronvolts)