Input interpretation

oxidants | molar mass
Summary
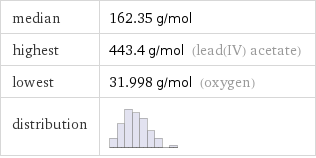
median | 162.35 g/mol highest | 443.4 g/mol (lead(IV) acetate) lowest | 31.998 g/mol (oxygen) distribution |
Units

Distribution plots
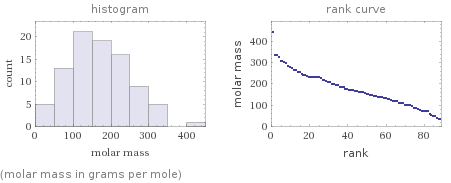
(molar mass in grams per mole)
Molar mass rankings
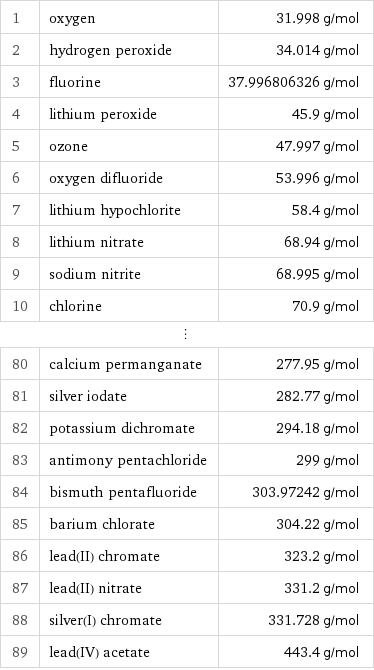
1 | oxygen | 31.998 g/mol 2 | hydrogen peroxide | 34.014 g/mol 3 | fluorine | 37.996806326 g/mol 4 | lithium peroxide | 45.9 g/mol 5 | ozone | 47.997 g/mol 6 | oxygen difluoride | 53.996 g/mol 7 | lithium hypochlorite | 58.4 g/mol 8 | lithium nitrate | 68.94 g/mol 9 | sodium nitrite | 68.995 g/mol 10 | chlorine | 70.9 g/mol ⋮ | | 80 | calcium permanganate | 277.95 g/mol 81 | silver iodate | 282.77 g/mol 82 | potassium dichromate | 294.18 g/mol 83 | antimony pentachloride | 299 g/mol 84 | bismuth pentafluoride | 303.97242 g/mol 85 | barium chlorate | 304.22 g/mol 86 | lead(II) chromate | 323.2 g/mol 87 | lead(II) nitrate | 331.2 g/mol 88 | silver(I) chromate | 331.728 g/mol 89 | lead(IV) acetate | 443.4 g/mol
Unit conversion for median molar mass 162.35 g/mol
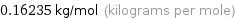
0.16235 kg/mol (kilograms per mole)
Comparisons for median molar mass 162.35 g/mol
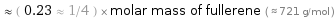
≈ ( 0.23 ≈ 1/4 ) × molar mass of fullerene ( ≈ 721 g/mol )
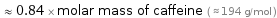
≈ 0.84 × molar mass of caffeine ( ≈ 194 g/mol )
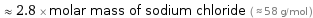
≈ 2.8 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities

Mass of a molecule m from m = M/N_A: | 2.7×10^-22 grams | 2.7×10^-25 kg (kilograms) | 162 u (unified atomic mass units) | 162 Da (daltons)
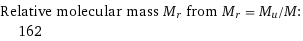
Relative molecular mass M_r from M_r = M_u/M: | 162