Input interpretation
silver(I) oxide
Chemical names and formulas
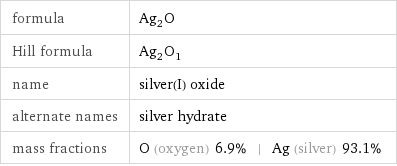
formula | Ag_2O Hill formula | Ag_2O_1 name | silver(I) oxide alternate names | silver hydrate mass fractions | O (oxygen) 6.9% | Ag (silver) 93.1%
Structure diagram
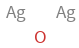
Structure diagram
Basic properties

molar mass | 231.7 g/mol
Units

Thermodynamic properties
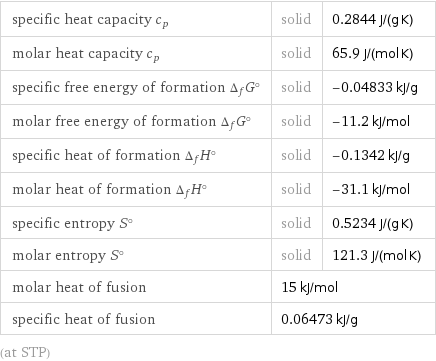
specific heat capacity c_p | solid | 0.2844 J/(g K) molar heat capacity c_p | solid | 65.9 J/(mol K) specific free energy of formation Δ_fG° | solid | -0.04833 kJ/g molar free energy of formation Δ_fG° | solid | -11.2 kJ/mol specific heat of formation Δ_fH° | solid | -0.1342 kJ/g molar heat of formation Δ_fH° | solid | -31.1 kJ/mol specific entropy S° | solid | 0.5234 J/(g K) molar entropy S° | solid | 121.3 J/(mol K) molar heat of fusion | 15 kJ/mol | specific heat of fusion | 0.06473 kJ/g | (at STP)
Chemical identifiers
![CAS number | 20667-12-3 SMILES identifier | [Ag].[Ag].[O] RTECS number | VW4900000 MDL number | MFCD00003404](../image_source/915565b1e266a58d10d593f2f9e58021.png)
CAS number | 20667-12-3 SMILES identifier | [Ag].[Ag].[O] RTECS number | VW4900000 MDL number | MFCD00003404