Input interpretation
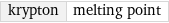
krypton | melting point
Result

-157.36 °C (degrees Celsius)
Unit conversions
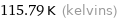
115.79 K (kelvins)
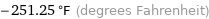
-251.25 °F (degrees Fahrenheit)
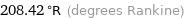
208.42 °R (degrees Rankine)

-125.89 °Ré (degrees Réaumur)

-75.114 °Rø (degrees Rømer)
Comparisons as temperature
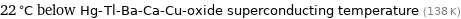
22 °C below Hg-Tl-Ba-Ca-Cu-oxide superconducting temperature (138 K)
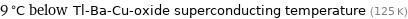
9 °C below Tl-Ba-Cu-oxide superconducting temperature (125 K)
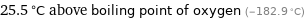
25.5 °C above boiling point of oxygen (-182.9 °C)
Corresponding quantities

Thermodynamic energy E from E = kT: | 10 meV (millielectronvolts)
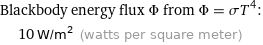
Blackbody energy flux Φ from Φ = σT^4: | 10 W/m^2 (watts per square meter)

Approximate luminous exitance from a planar blackbody radiator perpendicular to its surface: | 5.1×10^-65 lx (lux)
Thermodynamic properties
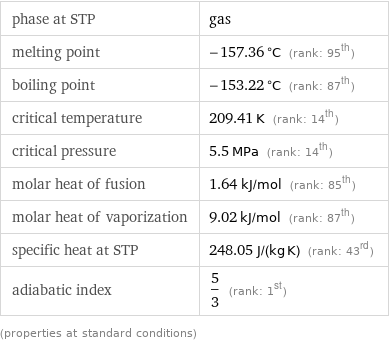
phase at STP | gas melting point | -157.36 °C (rank: 95th) boiling point | -153.22 °C (rank: 87th) critical temperature | 209.41 K (rank: 14th) critical pressure | 5.5 MPa (rank: 14th) molar heat of fusion | 1.64 kJ/mol (rank: 85th) molar heat of vaporization | 9.02 kJ/mol (rank: 87th) specific heat at STP | 248.05 J/(kg K) (rank: 43rd) adiabatic index | 5/3 (rank: 1st) (properties at standard conditions)