Input interpretation
strong acids
Members

trifluoromethanesulfonic acid | perchloric acid | hydrogen bromide | hydrogen iodide | hydrogen chloride | sulfuric acid | meldrum's acid | benzenesulfonic acid | methanesulfonic acid | nitric acid | isothiocyanic acid | 1, 3-cyclopentadiene | tropolone | chromic acid | trifluoroacetic acid | picric acid | iodic acid (total: 17)
Basic properties
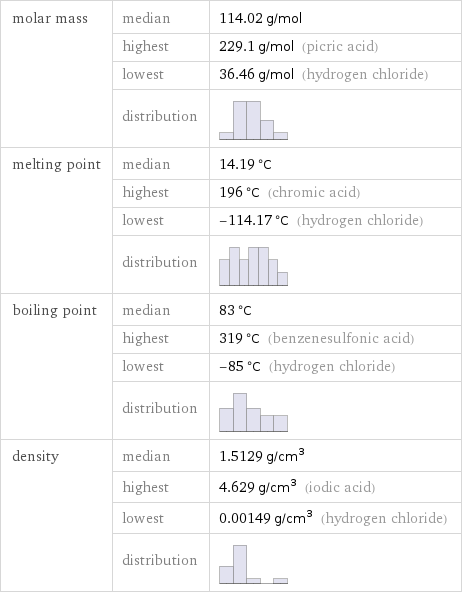
molar mass | median | 114.02 g/mol | highest | 229.1 g/mol (picric acid) | lowest | 36.46 g/mol (hydrogen chloride) | distribution | melting point | median | 14.19 °C | highest | 196 °C (chromic acid) | lowest | -114.17 °C (hydrogen chloride) | distribution | boiling point | median | 83 °C | highest | 319 °C (benzenesulfonic acid) | lowest | -85 °C (hydrogen chloride) | distribution | density | median | 1.5129 g/cm^3 | highest | 4.629 g/cm^3 (iodic acid) | lowest | 0.00149 g/cm^3 (hydrogen chloride) | distribution |
Units
