Input interpretation

fluorine | specific heat at STP
Result
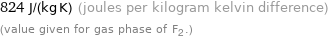
824 J/(kg K) (joules per kilogram kelvin difference) (value given for gas phase of F2.)
Unit conversions
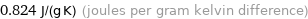
0.824 J/(g K) (joules per gram kelvin difference)
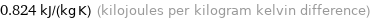
0.824 kJ/(kg K) (kilojoules per kilogram kelvin difference)
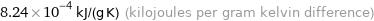
8.24×10^-4 kJ/(g K) (kilojoules per gram kelvin difference)
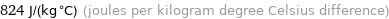
824 J/(kg °C) (joules per kilogram degree Celsius difference)
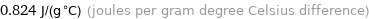
0.824 J/(g °C) (joules per gram degree Celsius difference)

0.197 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons
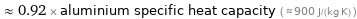
≈ 0.92 × aluminium specific heat capacity ( ≈ 900 J/(kg K) )
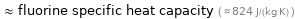
≈ fluorine specific heat capacity ( ≈ 824 J/(kg K) )
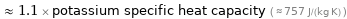
≈ 1.1 × potassium specific heat capacity ( ≈ 757 J/(kg K) )
Thermodynamic properties
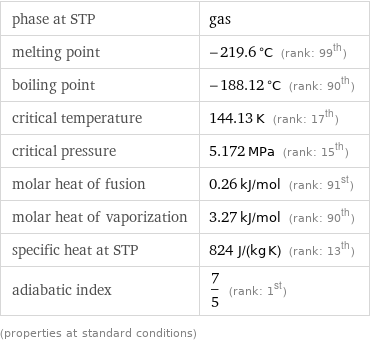
phase at STP | gas melting point | -219.6 °C (rank: 99th) boiling point | -188.12 °C (rank: 90th) critical temperature | 144.13 K (rank: 17th) critical pressure | 5.172 MPa (rank: 15th) molar heat of fusion | 0.26 kJ/mol (rank: 91st) molar heat of vaporization | 3.27 kJ/mol (rank: 90th) specific heat at STP | 824 J/(kg K) (rank: 13th) adiabatic index | 7/5 (rank: 1st) (properties at standard conditions)