Input interpretation

halogenated compounds | specific heat of vaporization
Summary
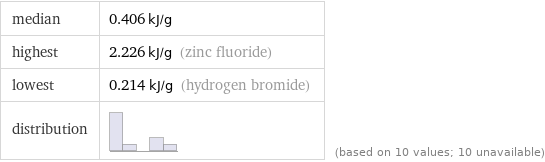
median | 0.406 kJ/g highest | 2.226 kJ/g (zinc fluoride) lowest | 0.214 kJ/g (hydrogen bromide) distribution | | (based on 10 values; 10 unavailable)
Entities with missing values
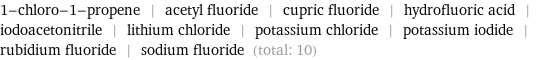
1-chloro-1-propene | acetyl fluoride | cupric fluoride | hydrofluoric acid | iodoacetonitrile | lithium chloride | potassium chloride | potassium iodide | rubidium fluoride | sodium fluoride (total: 10)
Distribution plots
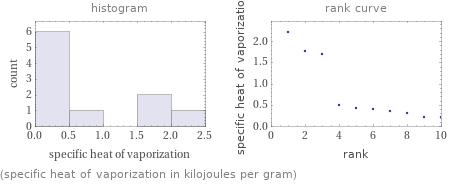
(specific heat of vaporization in kilojoules per gram)
Specific heat of vaporization rankings

1 | hydrogen bromide | 0.214 kJ/g 2 | 1, 1, 2, 2-tetrafluoroethane | 0.216 kJ/g 3 | fluorocyclohexane | 0.318 kJ/g 4 | chlorocyclopentane | 0.366 kJ/g 5 | methyl chloride | 0.398 kJ/g 6 | hydrogen chloride | 0.414 kJ/g 7 | fluoromethane | 0.505 kJ/g 8 | lithium bromide | 1.707 kJ/g 9 | sodium bromide | 1.757 kJ/g 10 | zinc fluoride | 2.226 kJ/g (based on 10 values; 10 unavailable)
Unit conversions for median specific heat of vaporization 0.406 kJ/g

406 J/g (joules per gram)
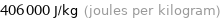
406000 J/kg (joules per kilogram)
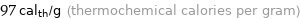
97 cal_th/g (thermochemical calories per gram)

97 kcal_th/kg (thermochemical kilocalories per kilogram)

0.097 kcal_th/g (thermochemical kilocalories per gram)

175 BTU_IT/lb (IT British thermal units per pound)