Input interpretation

alkali metals | nuclear radius
Summary
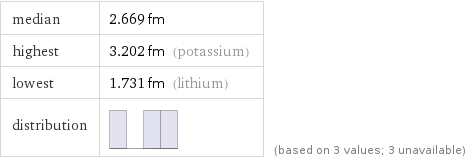
median | 2.669 fm highest | 3.202 fm (potassium) lowest | 1.731 fm (lithium) distribution | | (based on 3 values; 3 unavailable)
Entities with missing values
rubidium | cesium | francium (total: 3)
Ranked values

| | visual | ratios | 3 | lithium | | 0.5405 | 1 2 | sodium | | 0.8335 | 1.542 1 | potassium | | 1 | 1.85
Nuclear radius rankings

1 | lithium | 1.731 fm 2 | sodium | 2.669 fm 3 | potassium | 3.202 fm (based on 3 values; 3 unavailable)
Unit conversions for median nuclear radius 2.669 fm
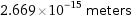
2.669×10^-15 meters
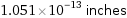
1.051×10^-13 inches
Comparisons for median nuclear radius 2.669 fm
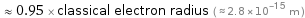
≈ 0.95 × classical electron radius ( ≈ 2.8×10^-15 m )
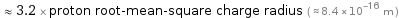
≈ 3.2 × proton root-mean-square charge radius ( ≈ 8.4×10^-16 m )