Input interpretation

ionic diatomics | element types
Summary
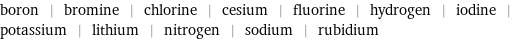
boron | bromine | chlorine | cesium | fluorine | hydrogen | iodine | potassium | lithium | nitrogen | sodium | rubidium
Periodic table location
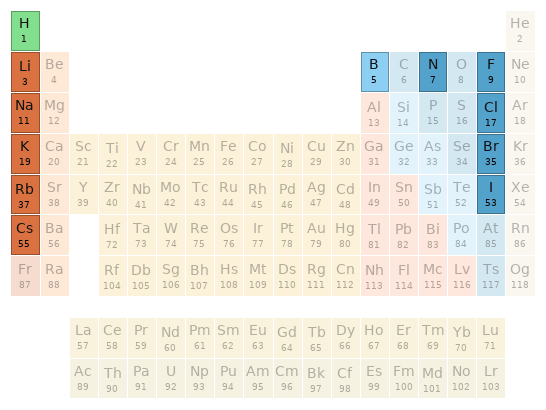
Periodic table location
Images

Images
Basic elemental properties
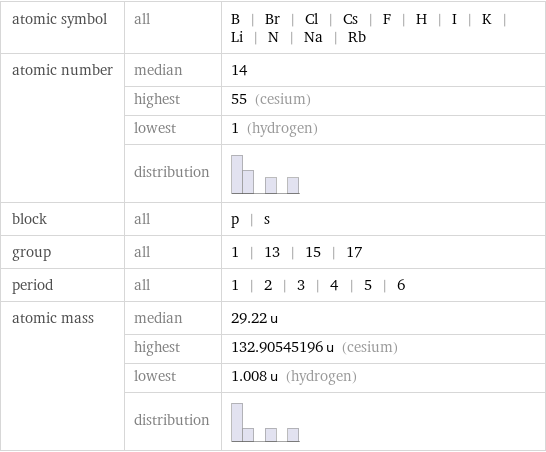
atomic symbol | all | B | Br | Cl | Cs | F | H | I | K | Li | N | Na | Rb atomic number | median | 14 | highest | 55 (cesium) | lowest | 1 (hydrogen) | distribution | block | all | p | s group | all | 1 | 13 | 15 | 17 period | all | 1 | 2 | 3 | 4 | 5 | 6 atomic mass | median | 29.22 u | highest | 132.90545196 u (cesium) | lowest | 1.008 u (hydrogen) | distribution |
Table

lithium hydride | hydrogen | lithium sodium hydride | hydrogen | sodium boron nitride | boron | nitrogen lithium chloride | chlorine | lithium potassium chloride | chlorine | potassium rubidium fluoride | fluorine | rubidium potassium bromide | bromine | potassium rubidium chloride | chlorine | rubidium lithium iodide | iodine | lithium cesium iodide | cesium | iodine
Thermodynamic properties
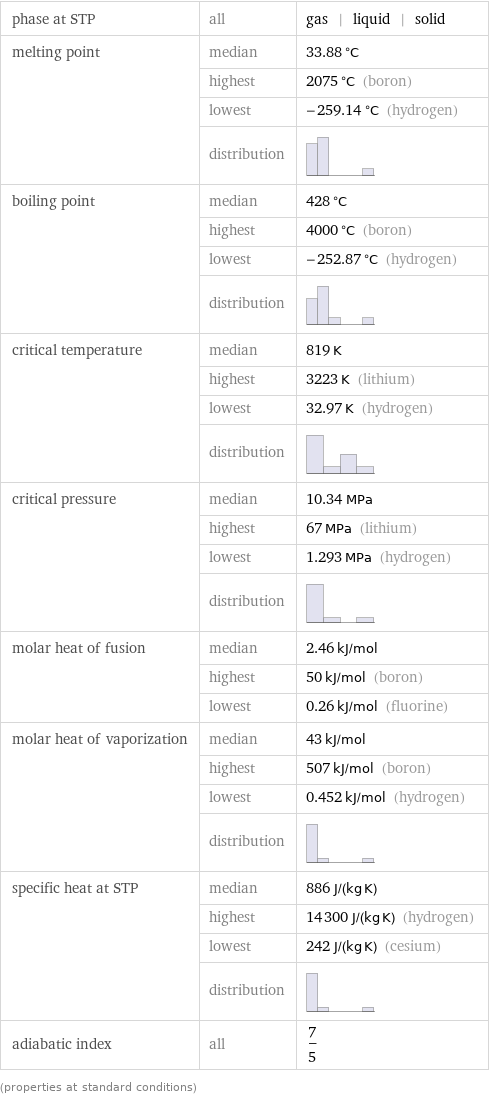
phase at STP | all | gas | liquid | solid melting point | median | 33.88 °C | highest | 2075 °C (boron) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | 428 °C | highest | 4000 °C (boron) | lowest | -252.87 °C (hydrogen) | distribution | critical temperature | median | 819 K | highest | 3223 K (lithium) | lowest | 32.97 K (hydrogen) | distribution | critical pressure | median | 10.34 MPa | highest | 67 MPa (lithium) | lowest | 1.293 MPa (hydrogen) | distribution | molar heat of fusion | median | 2.46 kJ/mol | highest | 50 kJ/mol (boron) | lowest | 0.26 kJ/mol (fluorine) molar heat of vaporization | median | 43 kJ/mol | highest | 507 kJ/mol (boron) | lowest | 0.452 kJ/mol (hydrogen) | distribution | specific heat at STP | median | 886 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 242 J/(kg K) (cesium) | distribution | adiabatic index | all | 7/5 (properties at standard conditions)
Material properties
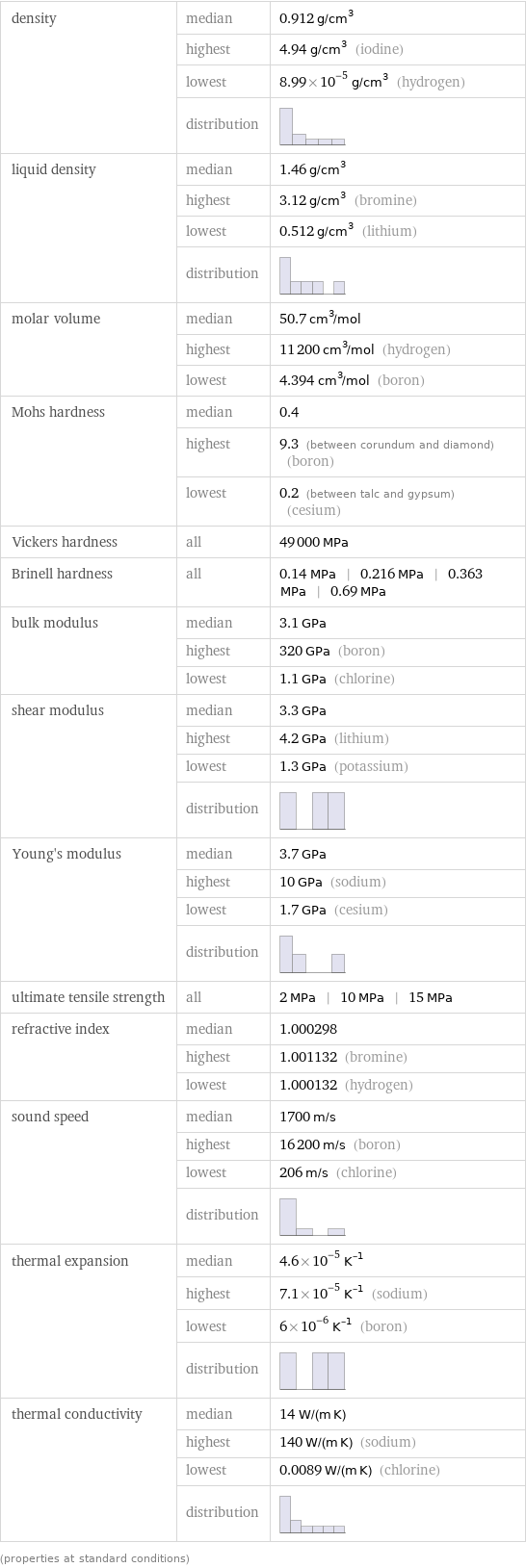
density | median | 0.912 g/cm^3 | highest | 4.94 g/cm^3 (iodine) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution | liquid density | median | 1.46 g/cm^3 | highest | 3.12 g/cm^3 (bromine) | lowest | 0.512 g/cm^3 (lithium) | distribution | molar volume | median | 50.7 cm^3/mol | highest | 11200 cm^3/mol (hydrogen) | lowest | 4.394 cm^3/mol (boron) Mohs hardness | median | 0.4 | highest | 9.3 (between corundum and diamond) (boron) | lowest | 0.2 (between talc and gypsum) (cesium) Vickers hardness | all | 49000 MPa Brinell hardness | all | 0.14 MPa | 0.216 MPa | 0.363 MPa | 0.69 MPa bulk modulus | median | 3.1 GPa | highest | 320 GPa (boron) | lowest | 1.1 GPa (chlorine) shear modulus | median | 3.3 GPa | highest | 4.2 GPa (lithium) | lowest | 1.3 GPa (potassium) | distribution | Young's modulus | median | 3.7 GPa | highest | 10 GPa (sodium) | lowest | 1.7 GPa (cesium) | distribution | ultimate tensile strength | all | 2 MPa | 10 MPa | 15 MPa refractive index | median | 1.000298 | highest | 1.001132 (bromine) | lowest | 1.000132 (hydrogen) sound speed | median | 1700 m/s | highest | 16200 m/s (boron) | lowest | 206 m/s (chlorine) | distribution | thermal expansion | median | 4.6×10^-5 K^(-1) | highest | 7.1×10^-5 K^(-1) (sodium) | lowest | 6×10^-6 K^(-1) (boron) | distribution | thermal conductivity | median | 14 W/(m K) | highest | 140 W/(m K) (sodium) | lowest | 0.0089 W/(m K) (chlorine) | distribution | (properties at standard conditions)
Electromagnetic properties
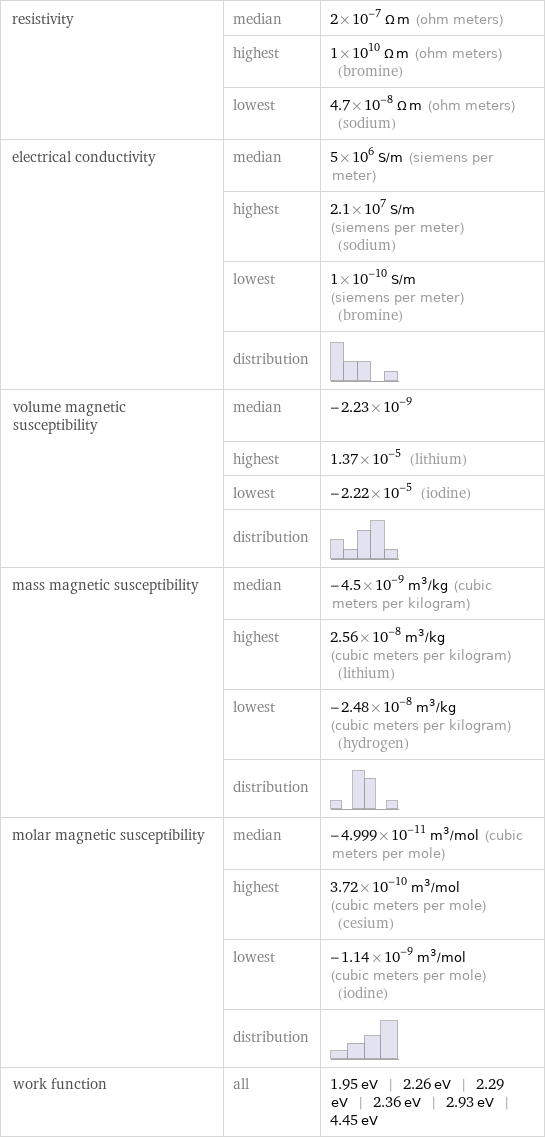
resistivity | median | 2×10^-7 Ω m (ohm meters) | highest | 1×10^10 Ω m (ohm meters) (bromine) | lowest | 4.7×10^-8 Ω m (ohm meters) (sodium) electrical conductivity | median | 5×10^6 S/m (siemens per meter) | highest | 2.1×10^7 S/m (siemens per meter) (sodium) | lowest | 1×10^-10 S/m (siemens per meter) (bromine) | distribution | volume magnetic susceptibility | median | -2.23×10^-9 | highest | 1.37×10^-5 (lithium) | lowest | -2.22×10^-5 (iodine) | distribution | mass magnetic susceptibility | median | -4.5×10^-9 m^3/kg (cubic meters per kilogram) | highest | 2.56×10^-8 m^3/kg (cubic meters per kilogram) (lithium) | lowest | -2.48×10^-8 m^3/kg (cubic meters per kilogram) (hydrogen) | distribution | molar magnetic susceptibility | median | -4.999×10^-11 m^3/mol (cubic meters per mole) | highest | 3.72×10^-10 m^3/mol (cubic meters per mole) (cesium) | lowest | -1.14×10^-9 m^3/mol (cubic meters per mole) (iodine) | distribution | work function | all | 1.95 eV | 2.26 eV | 2.29 eV | 2.36 eV | 2.93 eV | 4.45 eV
Reactivity
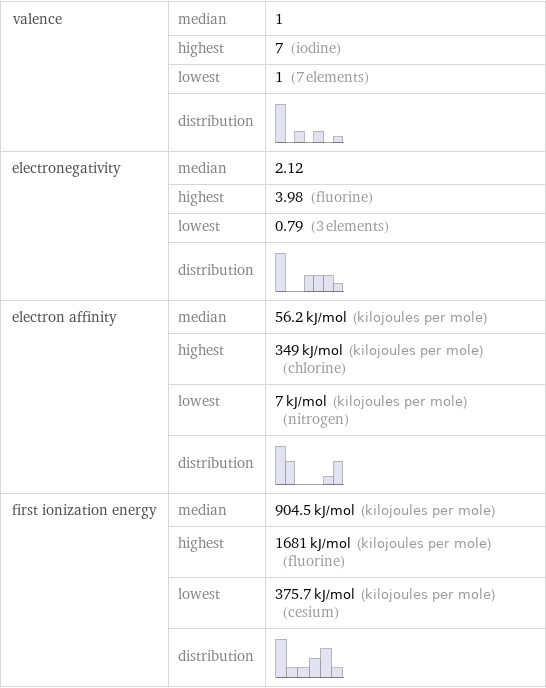
valence | median | 1 | highest | 7 (iodine) | lowest | 1 (7 elements) | distribution | electronegativity | median | 2.12 | highest | 3.98 (fluorine) | lowest | 0.79 (3 elements) | distribution | electron affinity | median | 56.2 kJ/mol (kilojoules per mole) | highest | 349 kJ/mol (kilojoules per mole) (chlorine) | lowest | 7 kJ/mol (kilojoules per mole) (nitrogen) | distribution | first ionization energy | median | 904.5 kJ/mol (kilojoules per mole) | highest | 1681 kJ/mol (kilojoules per mole) (fluorine) | lowest | 375.7 kJ/mol (kilojoules per mole) (cesium) | distribution |
Atomic properties
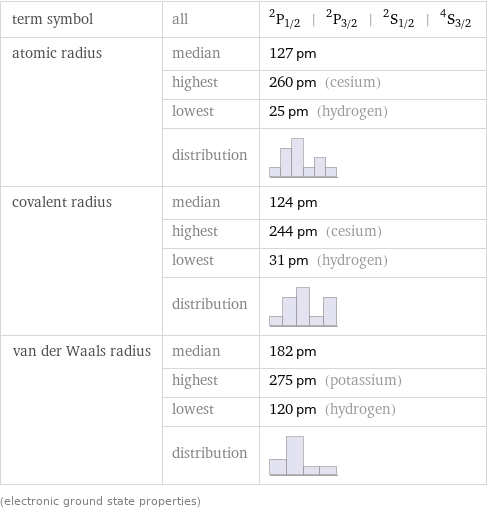
term symbol | all | ^2P_(1/2) | ^2P_(3/2) | ^2S_(1/2) | ^4S_(3/2) atomic radius | median | 127 pm | highest | 260 pm (cesium) | lowest | 25 pm (hydrogen) | distribution | covalent radius | median | 124 pm | highest | 244 pm (cesium) | lowest | 31 pm (hydrogen) | distribution | van der Waals radius | median | 182 pm | highest | 275 pm (potassium) | lowest | 120 pm (hydrogen) | distribution | (electronic ground state properties)