Input interpretation
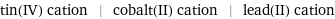
tin(IV) cation | cobalt(II) cation | lead(II) cation
Structure diagrams

Structure diagrams
General properties
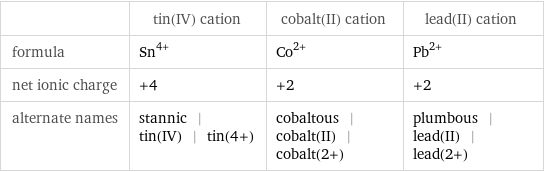
| tin(IV) cation | cobalt(II) cation | lead(II) cation formula | Sn^(4+) | Co^(2+) | Pb^(2+) net ionic charge | +4 | +2 | +2 alternate names | stannic | tin(IV) | tin(4+) | cobaltous | cobalt(II) | cobalt(2+) | plumbous | lead(II) | lead(2+)
Ionic radii
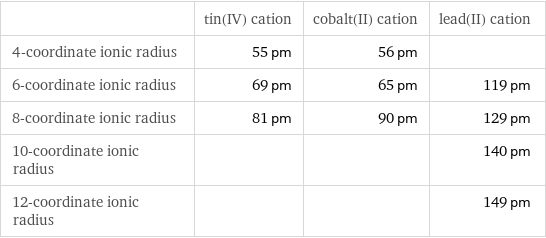
| tin(IV) cation | cobalt(II) cation | lead(II) cation 4-coordinate ionic radius | 55 pm | 56 pm | 6-coordinate ionic radius | 69 pm | 65 pm | 119 pm 8-coordinate ionic radius | 81 pm | 90 pm | 129 pm 10-coordinate ionic radius | | | 140 pm 12-coordinate ionic radius | | | 149 pm
Units

Other properties
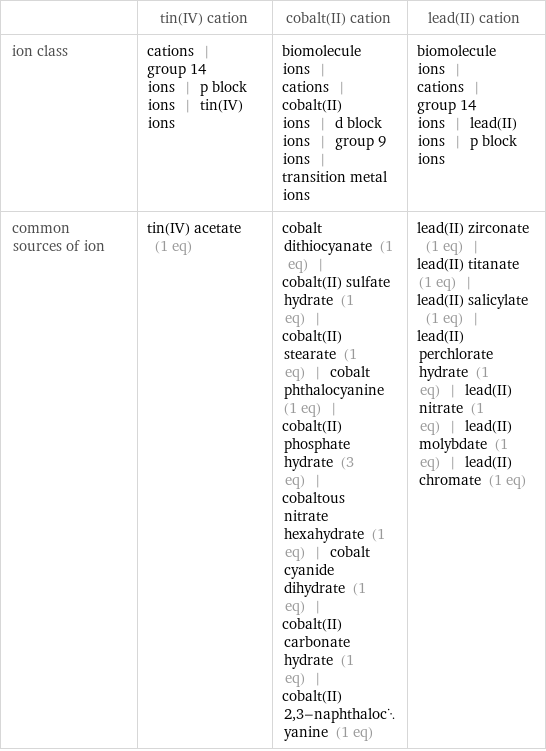
| tin(IV) cation | cobalt(II) cation | lead(II) cation ion class | cations | group 14 ions | p block ions | tin(IV) ions | biomolecule ions | cations | cobalt(II) ions | d block ions | group 9 ions | transition metal ions | biomolecule ions | cations | group 14 ions | lead(II) ions | p block ions common sources of ion | tin(IV) acetate (1 eq) | cobalt dithiocyanate (1 eq) | cobalt(II) sulfate hydrate (1 eq) | cobalt(II) stearate (1 eq) | cobalt phthalocyanine (1 eq) | cobalt(II) phosphate hydrate (3 eq) | cobaltous nitrate hexahydrate (1 eq) | cobalt cyanide dihydrate (1 eq) | cobalt(II) carbonate hydrate (1 eq) | cobalt(II) 2, 3-naphthalocyanine (1 eq) | lead(II) zirconate (1 eq) | lead(II) titanate (1 eq) | lead(II) salicylate (1 eq) | lead(II) perchlorate hydrate (1 eq) | lead(II) nitrate (1 eq) | lead(II) molybdate (1 eq) | lead(II) chromate (1 eq)