Input interpretation

strong electrolytes | proton count
Summary
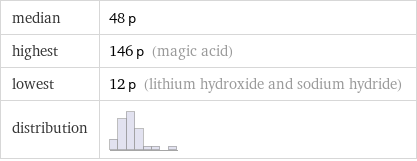
median | 48 p highest | 146 p (magic acid) lowest | 12 p (lithium hydroxide and sodium hydride) distribution |
Units

Distribution plots
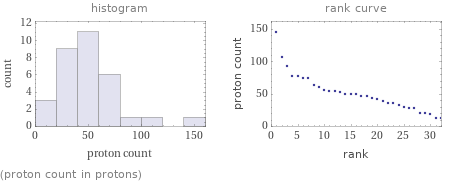
(proton count in protons)
Proton count rankings
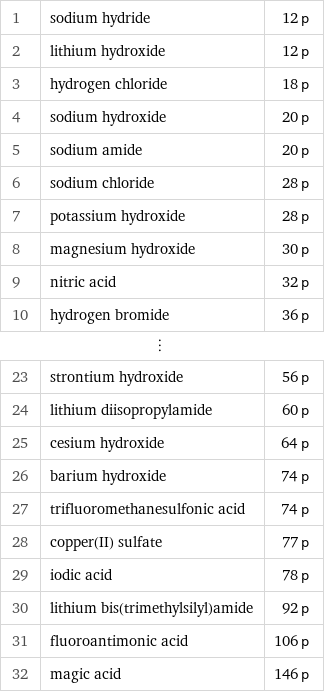
1 | sodium hydride | 12 p 2 | lithium hydroxide | 12 p 3 | hydrogen chloride | 18 p 4 | sodium hydroxide | 20 p 5 | sodium amide | 20 p 6 | sodium chloride | 28 p 7 | potassium hydroxide | 28 p 8 | magnesium hydroxide | 30 p 9 | nitric acid | 32 p 10 | hydrogen bromide | 36 p ⋮ | | 23 | strontium hydroxide | 56 p 24 | lithium diisopropylamide | 60 p 25 | cesium hydroxide | 64 p 26 | barium hydroxide | 74 p 27 | trifluoromethanesulfonic acid | 74 p 28 | copper(II) sulfate | 77 p 29 | iodic acid | 78 p 30 | lithium bis(trimethylsilyl)amide | 92 p 31 | fluoroantimonic acid | 106 p 32 | magic acid | 146 p
Corresponding quantity

Moles of protons from n = N/N_A: | 8×10^-23 mol (moles)