Input interpretation
nitrogen trioxide | sodium dithionate dihydrate
Chemical names and formulas
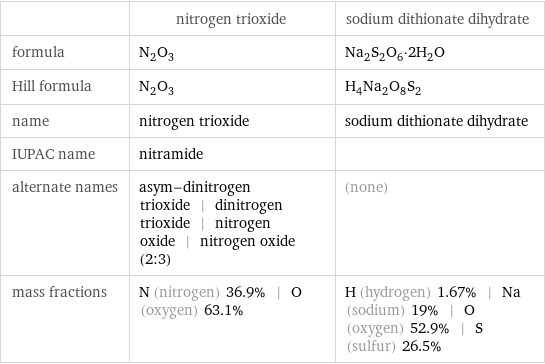
| nitrogen trioxide | sodium dithionate dihydrate formula | N_2O_3 | Na_2S_2O_6·2H_2O Hill formula | N_2O_3 | H_4Na_2O_8S_2 name | nitrogen trioxide | sodium dithionate dihydrate IUPAC name | nitramide | alternate names | asym-dinitrogen trioxide | dinitrogen trioxide | nitrogen oxide | nitrogen oxide (2:3) | (none) mass fractions | N (nitrogen) 36.9% | O (oxygen) 63.1% | H (hydrogen) 1.67% | Na (sodium) 19% | O (oxygen) 52.9% | S (sulfur) 26.5%
Structure diagrams
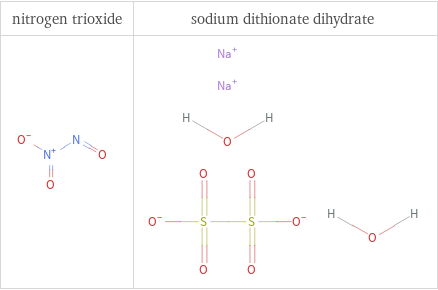
Structure diagrams
3D structure
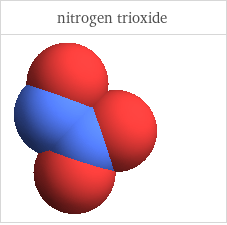
3D structure
Basic properties
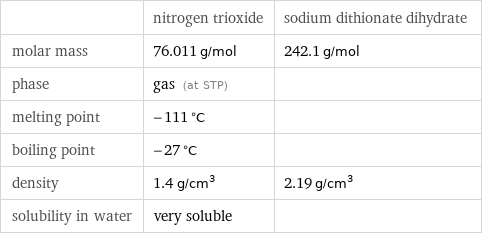
| nitrogen trioxide | sodium dithionate dihydrate molar mass | 76.011 g/mol | 242.1 g/mol phase | gas (at STP) | melting point | -111 °C | boiling point | -27 °C | density | 1.4 g/cm^3 | 2.19 g/cm^3 solubility in water | very soluble |
Units

Gas properties

| nitrogen trioxide density | 1.4 g/cm^3 molar volume | 54 cm^3/mol
Units
