Input interpretation

group 13 elements | boiling point
Summary
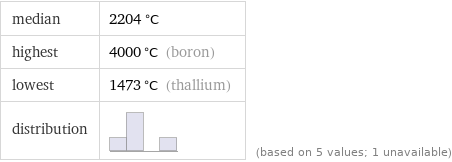
median | 2204 °C highest | 4000 °C (boron) lowest | 1473 °C (thallium) distribution | | (based on 5 values; 1 unavailable)
Entity with missing value
nihonium
Plot
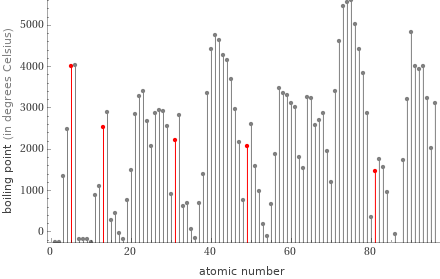
Plot
Boiling point rankings
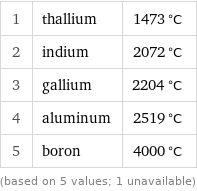
1 | thallium | 1473 °C 2 | indium | 2072 °C 3 | gallium | 2204 °C 4 | aluminum | 2519 °C 5 | boron | 4000 °C (based on 5 values; 1 unavailable)
Unit conversions for median boiling point 2204 °C
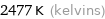
2477 K (kelvins)

3999 °F (degrees Fahrenheit)
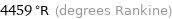
4459 °R (degrees Rankine)

1763 °Ré (degrees Réaumur)
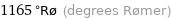
1165 °Rø (degrees Rømer)
Comparison for median boiling point 2204 °C
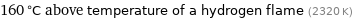
160 °C above temperature of a hydrogen flame (2320 K)
Blackbody information for median boiling point 2204 °C (degrees Celsius)
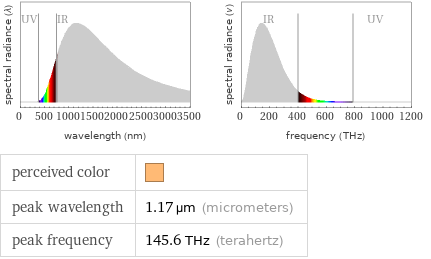
perceived color | peak wavelength | 1.17 µm (micrometers) peak frequency | 145.6 THz (terahertz)
Corresponding quantities
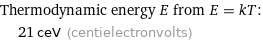
Thermodynamic energy E from E = kT: | 21 ceV (centielectronvolts)
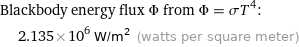
Blackbody energy flux Φ from Φ = σT^4: | 2.135×10^6 W/m^2 (watts per square meter)

Approximate luminous exitance from a planar blackbody radiator perpendicular to its surface: | 1.611×10^7 lx (lux)