Input interpretation
rubidium (chemical element) | radium (chemical element) | beryllium (chemical element)
Periodic table location
Periodic table location
Images
Images
Basic elemental properties
![| rubidium | radium | beryllium atomic symbol | Rb | Ra | Be atomic number | 37 | 88 | 4 short electronic configuration | [Kr]5s^1 | [Rn]7s^2 | [He]2s^2 Aufbau diagram | 5s | 7s | 2s block | s | s | s group | 1 | 2 | 2 period | 5 | 7 | 2 atomic mass | 85.4678 u | 226 u | 9.0121831 u half-life | (stable) | 1600 yr | (stable)](../image_source/4acd24237599ceaf3a715ca88fab3eb1.png)
| rubidium | radium | beryllium atomic symbol | Rb | Ra | Be atomic number | 37 | 88 | 4 short electronic configuration | [Kr]5s^1 | [Rn]7s^2 | [He]2s^2 Aufbau diagram | 5s | 7s | 2s block | s | s | s group | 1 | 2 | 2 period | 5 | 7 | 2 atomic mass | 85.4678 u | 226 u | 9.0121831 u half-life | (stable) | 1600 yr | (stable)
Thermodynamic properties
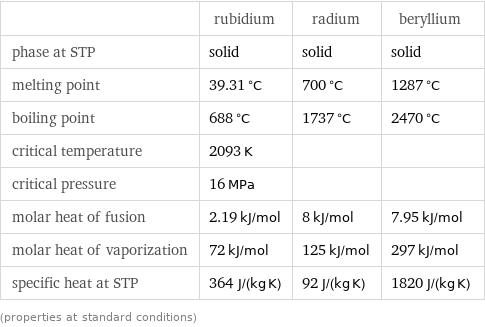
| rubidium | radium | beryllium phase at STP | solid | solid | solid melting point | 39.31 °C | 700 °C | 1287 °C boiling point | 688 °C | 1737 °C | 2470 °C critical temperature | 2093 K | | critical pressure | 16 MPa | | molar heat of fusion | 2.19 kJ/mol | 8 kJ/mol | 7.95 kJ/mol molar heat of vaporization | 72 kJ/mol | 125 kJ/mol | 297 kJ/mol specific heat at STP | 364 J/(kg K) | 92 J/(kg K) | 1820 J/(kg K) (properties at standard conditions)
Material properties
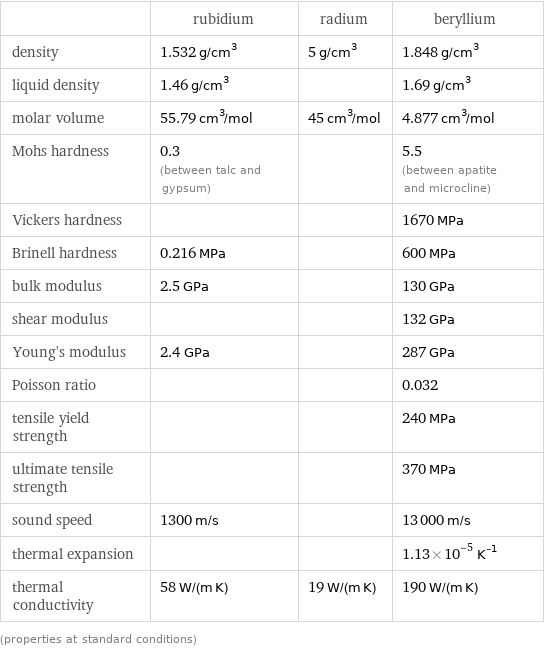
| rubidium | radium | beryllium density | 1.532 g/cm^3 | 5 g/cm^3 | 1.848 g/cm^3 liquid density | 1.46 g/cm^3 | | 1.69 g/cm^3 molar volume | 55.79 cm^3/mol | 45 cm^3/mol | 4.877 cm^3/mol Mohs hardness | 0.3 (between talc and gypsum) | | 5.5 (between apatite and microcline) Vickers hardness | | | 1670 MPa Brinell hardness | 0.216 MPa | | 600 MPa bulk modulus | 2.5 GPa | | 130 GPa shear modulus | | | 132 GPa Young's modulus | 2.4 GPa | | 287 GPa Poisson ratio | | | 0.032 tensile yield strength | | | 240 MPa ultimate tensile strength | | | 370 MPa sound speed | 1300 m/s | | 13000 m/s thermal expansion | | | 1.13×10^-5 K^(-1) thermal conductivity | 58 W/(m K) | 19 W/(m K) | 190 W/(m K) (properties at standard conditions)
Electromagnetic properties
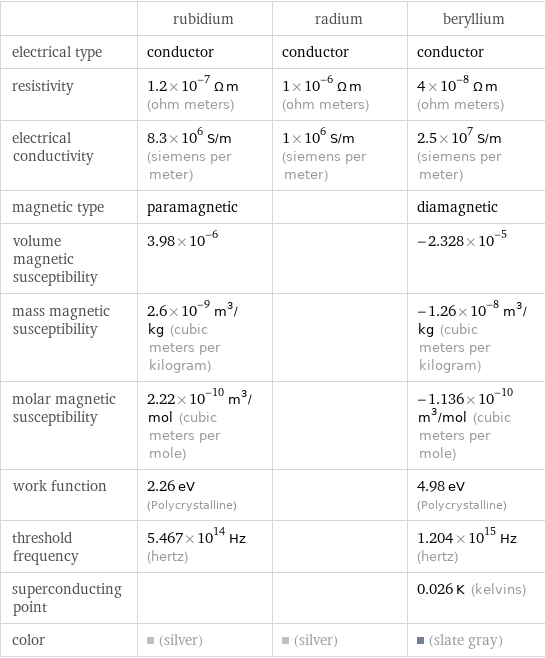
| rubidium | radium | beryllium electrical type | conductor | conductor | conductor resistivity | 1.2×10^-7 Ω m (ohm meters) | 1×10^-6 Ω m (ohm meters) | 4×10^-8 Ω m (ohm meters) electrical conductivity | 8.3×10^6 S/m (siemens per meter) | 1×10^6 S/m (siemens per meter) | 2.5×10^7 S/m (siemens per meter) magnetic type | paramagnetic | | diamagnetic volume magnetic susceptibility | 3.98×10^-6 | | -2.328×10^-5 mass magnetic susceptibility | 2.6×10^-9 m^3/kg (cubic meters per kilogram) | | -1.26×10^-8 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | 2.22×10^-10 m^3/mol (cubic meters per mole) | | -1.136×10^-10 m^3/mol (cubic meters per mole) work function | 2.26 eV (Polycrystalline) | | 4.98 eV (Polycrystalline) threshold frequency | 5.467×10^14 Hz (hertz) | | 1.204×10^15 Hz (hertz) superconducting point | | | 0.026 K (kelvins) color | (silver) | (silver) | (slate gray)
Reactivity

| rubidium | radium | beryllium valence | 1 | 2 | 2 electronegativity | 0.82 | 0.9 | 1.57 electron affinity | 46.9 kJ/mol (kilojoules per mole) | | 0 kJ/mol (kilojoules per mole) first ionization energy | 403 kJ/mol (kilojoules per mole) | 509.3 kJ/mol (kilojoules per mole) | 899.5 kJ/mol (kilojoules per mole) ionization energies | 403 kJ/mol | 2633 kJ/mol | 3860 kJ/mol | 5080 kJ/mol | 6850 kJ/mol | 8140 kJ/mol | 9570 kJ/mol | 13120 kJ/mol | 14500 kJ/mol | 26740 kJ/mol | 509.3 kJ/mol | 979 kJ/mol | 899.5 kJ/mol | 1757.1 kJ/mol | 14848.7 kJ/mol | 21006.6 kJ/mol
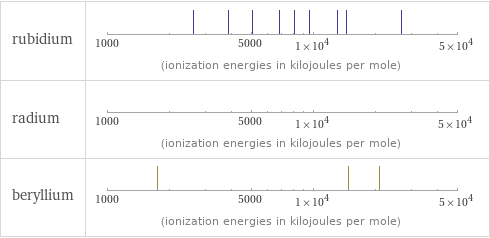
Reactivity
Atomic properties

| rubidium | radium | beryllium term symbol | ^2S_(1/2) | ^1S_0 | ^1S_0 atomic radius | 235 pm | 215 pm | 105 pm covalent radius | 220 pm | 221 pm | 96 pm (electronic ground state properties)
Abundances
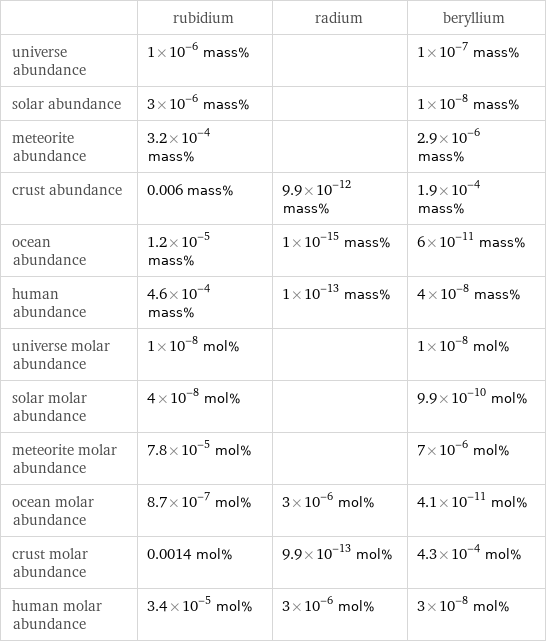
| rubidium | radium | beryllium universe abundance | 1×10^-6 mass% | | 1×10^-7 mass% solar abundance | 3×10^-6 mass% | | 1×10^-8 mass% meteorite abundance | 3.2×10^-4 mass% | | 2.9×10^-6 mass% crust abundance | 0.006 mass% | 9.9×10^-12 mass% | 1.9×10^-4 mass% ocean abundance | 1.2×10^-5 mass% | 1×10^-15 mass% | 6×10^-11 mass% human abundance | 4.6×10^-4 mass% | 1×10^-13 mass% | 4×10^-8 mass% universe molar abundance | 1×10^-8 mol% | | 1×10^-8 mol% solar molar abundance | 4×10^-8 mol% | | 9.9×10^-10 mol% meteorite molar abundance | 7.8×10^-5 mol% | | 7×10^-6 mol% ocean molar abundance | 8.7×10^-7 mol% | 3×10^-6 mol% | 4.1×10^-11 mol% crust molar abundance | 0.0014 mol% | 9.9×10^-13 mol% | 4.3×10^-4 mol% human molar abundance | 3.4×10^-5 mol% | 3×10^-6 mol% | 3×10^-8 mol%
Nuclear properties

| rubidium | radium | beryllium half-life | (stable) | 1600 yr | (stable) specific radioactivity | | 36.5 GBq/g | decay mode | | alpha emission | stable isotopes | Rb-85 (72.17%) | | Be-9 (100%) unstable isotopes | Rb-87 (48.1 Gyr) | ... | Ra-226 (1600 yr) | ... | Be-10 (1.51 Myr) | ...
Units

Identifiers

| rubidium | radium | beryllium CAS number | 7440-17-7 | 7440-14-4 | 7440-41-7 PubChem CID number | 5357696 | 6328144 | 5460467 Gmelin number | | 40437 |