Input interpretation
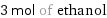
3 mol of ethanol
Basic properties for 3 mol
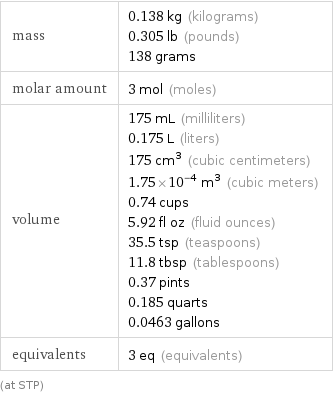
mass | 0.138 kg (kilograms) 0.305 lb (pounds) 138 grams molar amount | 3 mol (moles) volume | 175 mL (milliliters) 0.175 L (liters) 175 cm^3 (cubic centimeters) 1.75×10^-4 m^3 (cubic meters) 0.74 cups 5.92 fl oz (fluid ounces) 35.5 tsp (teaspoons) 11.8 tbsp (tablespoons) 0.37 pints 0.185 quarts 0.0463 gallons equivalents | 3 eq (equivalents) (at STP)
Thermodynamic properties for 3 mol
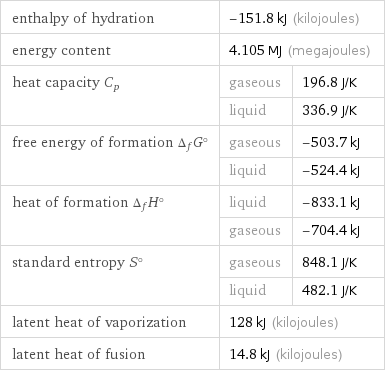
enthalpy of hydration | -151.8 kJ (kilojoules) | energy content | 4.105 MJ (megajoules) | heat capacity C_p | gaseous | 196.8 J/K | liquid | 336.9 J/K free energy of formation Δ_fG° | gaseous | -503.7 kJ | liquid | -524.4 kJ heat of formation Δ_fH° | liquid | -833.1 kJ | gaseous | -704.4 kJ standard entropy S° | gaseous | 848.1 J/K | liquid | 482.1 J/K latent heat of vaporization | 128 kJ (kilojoules) | latent heat of fusion | 14.8 kJ (kilojoules) |
Units

Energy vs. temperature for 3 mol
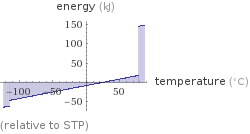
(relative to STP)
Units

Phase change energies for 3 mol from 25 °C

energy required to heat to boiling point | 17.9 kJ (kilojoules) energy required to convert to vapor | 128 kJ (kilojoules) energy required to heat to boiling point and convert to vapor | 146 kJ (kilojoules) energy released from cooling to freezing point | 46.8 kJ (kilojoules) energy released from converting to solid | 14.8 kJ (kilojoules) energy released from cooling to freezing point and converting to solid | 61.6 kJ (kilojoules)
Corresponding quantities
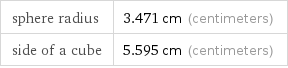
sphere radius | 3.471 cm (centimeters) side of a cube | 5.595 cm (centimeters)
Mass composition for 3 mol
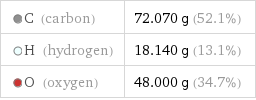
C (carbon) | 72.070 g (52.1%) H (hydrogen) | 18.140 g (13.1%) O (oxygen) | 48.000 g (34.7%)
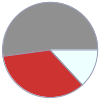
Mass composition for 3 mol
Lewis structure
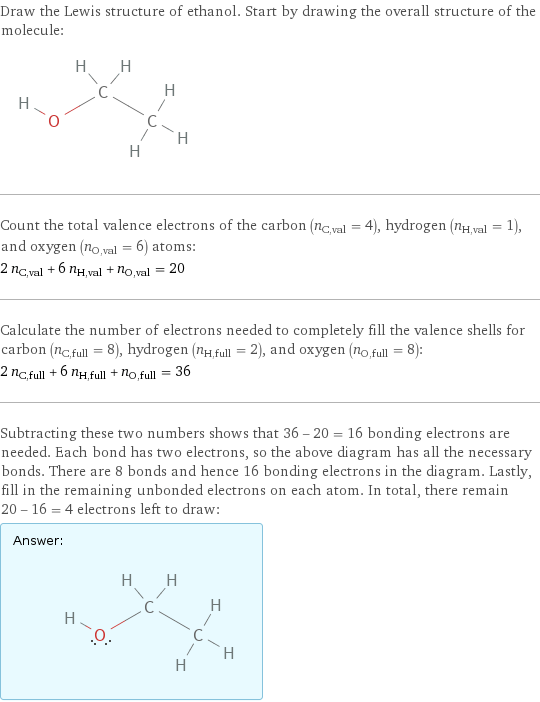
Draw the Lewis structure of ethanol. Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), and oxygen (n_O, val = 6) atoms: 2 n_C, val + 6 n_H, val + n_O, val = 20 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), and oxygen (n_O, full = 8): 2 n_C, full + 6 n_H, full + n_O, full = 36 Subtracting these two numbers shows that 36 - 20 = 16 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 8 bonds and hence 16 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 20 - 16 = 4 electrons left to draw: Answer: | |
Chemical names and formulas

formula | CH_3CH_2OH Hill formula | C_2H_6O name | ethanol IUPAC name | ethanol
Substance properties
molar mass | 46.07 g/mol phase | liquid (at STP) melting point | -114 °C boiling point | 78 °C density | 0.789 g/cm^3 solubility in water | miscible surface tension | 0.02275 N/m dynamic viscosity | 0.001074 Pa s (at 25 °C) odor threshold | 350 ppm
Units
