Input interpretation
alkaline earth metals
Members
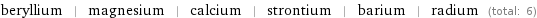
beryllium | magnesium | calcium | strontium | barium | radium (total: 6)
Periodic table location
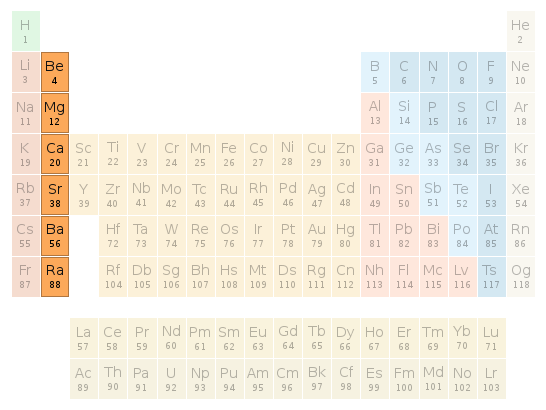
Periodic table location
Images
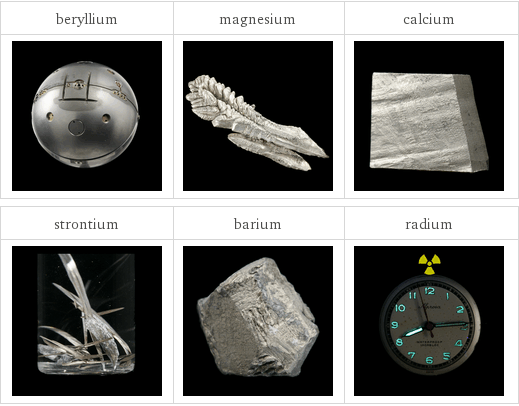
Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration beryllium | Be | 4 | [He]2s^2 magnesium | Mg | 12 | [Ne]3s^2 calcium | Ca | 20 | [Ar]4s^2 strontium | Sr | 38 | [Kr]5s^2 barium | Ba | 56 | [Xe]6s^2 radium | Ra | 88 | [Rn]7s^2 | Aufbau diagram | block | group beryllium | 2s | s | 2 magnesium | 3s | s | 2 calcium | 4s | s | 2 strontium | 5s | s | 2 barium | 6s | s | 2 radium | 7s | s | 2 | period | atomic mass | half-life beryllium | 2 | 9.0121831 u | (stable) magnesium | 3 | 24.305 u | (stable) calcium | 4 | 40.078 u | (stable) strontium | 5 | 87.62 u | (stable) barium | 6 | 137.327 u | (stable) radium | 7 | 226 u | 1600 yr](../image_source/c4da7e4a9aafb88e1b740e01220de2ac.png)
| atomic symbol | atomic number | short electronic configuration beryllium | Be | 4 | [He]2s^2 magnesium | Mg | 12 | [Ne]3s^2 calcium | Ca | 20 | [Ar]4s^2 strontium | Sr | 38 | [Kr]5s^2 barium | Ba | 56 | [Xe]6s^2 radium | Ra | 88 | [Rn]7s^2 | Aufbau diagram | block | group beryllium | 2s | s | 2 magnesium | 3s | s | 2 calcium | 4s | s | 2 strontium | 5s | s | 2 barium | 6s | s | 2 radium | 7s | s | 2 | period | atomic mass | half-life beryllium | 2 | 9.0121831 u | (stable) magnesium | 3 | 24.305 u | (stable) calcium | 4 | 40.078 u | (stable) strontium | 5 | 87.62 u | (stable) barium | 6 | 137.327 u | (stable) radium | 7 | 226 u | 1600 yr
Thermodynamic properties
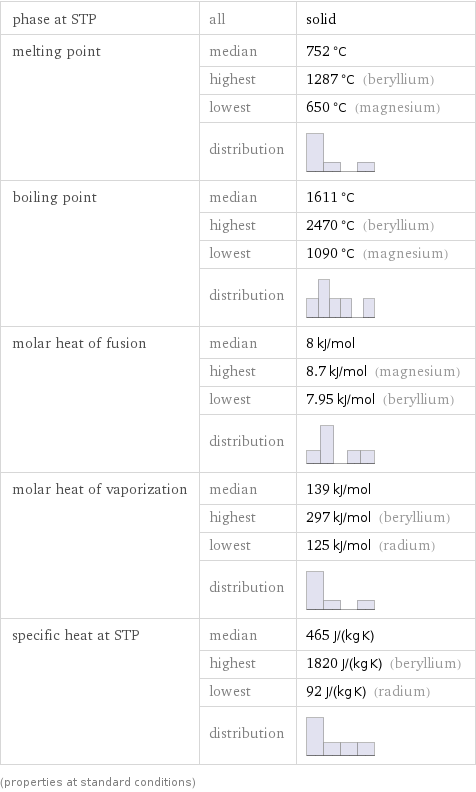
phase at STP | all | solid melting point | median | 752 °C | highest | 1287 °C (beryllium) | lowest | 650 °C (magnesium) | distribution | boiling point | median | 1611 °C | highest | 2470 °C (beryllium) | lowest | 1090 °C (magnesium) | distribution | molar heat of fusion | median | 8 kJ/mol | highest | 8.7 kJ/mol (magnesium) | lowest | 7.95 kJ/mol (beryllium) | distribution | molar heat of vaporization | median | 139 kJ/mol | highest | 297 kJ/mol (beryllium) | lowest | 125 kJ/mol (radium) | distribution | specific heat at STP | median | 465 J/(kg K) | highest | 1820 J/(kg K) (beryllium) | lowest | 92 J/(kg K) (radium) | distribution | (properties at standard conditions)
Material properties
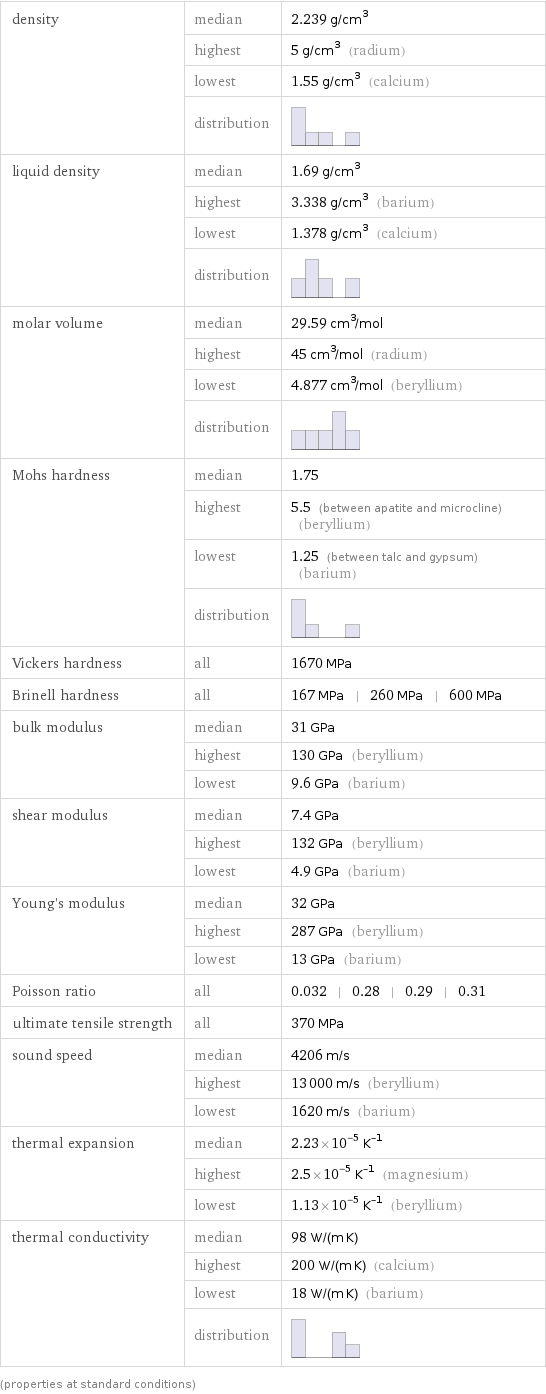
density | median | 2.239 g/cm^3 | highest | 5 g/cm^3 (radium) | lowest | 1.55 g/cm^3 (calcium) | distribution | liquid density | median | 1.69 g/cm^3 | highest | 3.338 g/cm^3 (barium) | lowest | 1.378 g/cm^3 (calcium) | distribution | molar volume | median | 29.59 cm^3/mol | highest | 45 cm^3/mol (radium) | lowest | 4.877 cm^3/mol (beryllium) | distribution | Mohs hardness | median | 1.75 | highest | 5.5 (between apatite and microcline) (beryllium) | lowest | 1.25 (between talc and gypsum) (barium) | distribution | Vickers hardness | all | 1670 MPa Brinell hardness | all | 167 MPa | 260 MPa | 600 MPa bulk modulus | median | 31 GPa | highest | 130 GPa (beryllium) | lowest | 9.6 GPa (barium) shear modulus | median | 7.4 GPa | highest | 132 GPa (beryllium) | lowest | 4.9 GPa (barium) Young's modulus | median | 32 GPa | highest | 287 GPa (beryllium) | lowest | 13 GPa (barium) Poisson ratio | all | 0.032 | 0.28 | 0.29 | 0.31 ultimate tensile strength | all | 370 MPa sound speed | median | 4206 m/s | highest | 13000 m/s (beryllium) | lowest | 1620 m/s (barium) thermal expansion | median | 2.23×10^-5 K^(-1) | highest | 2.5×10^-5 K^(-1) (magnesium) | lowest | 1.13×10^-5 K^(-1) (beryllium) thermal conductivity | median | 98 W/(m K) | highest | 200 W/(m K) (calcium) | lowest | 18 W/(m K) (barium) | distribution | (properties at standard conditions)
Electromagnetic properties
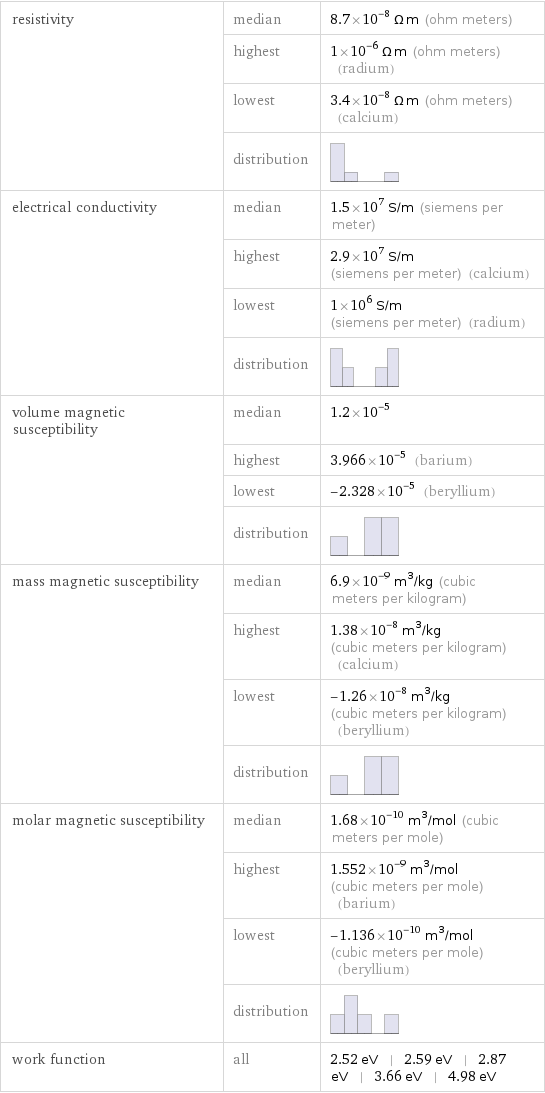
resistivity | median | 8.7×10^-8 Ω m (ohm meters) | highest | 1×10^-6 Ω m (ohm meters) (radium) | lowest | 3.4×10^-8 Ω m (ohm meters) (calcium) | distribution | electrical conductivity | median | 1.5×10^7 S/m (siemens per meter) | highest | 2.9×10^7 S/m (siemens per meter) (calcium) | lowest | 1×10^6 S/m (siemens per meter) (radium) | distribution | volume magnetic susceptibility | median | 1.2×10^-5 | highest | 3.966×10^-5 (barium) | lowest | -2.328×10^-5 (beryllium) | distribution | mass magnetic susceptibility | median | 6.9×10^-9 m^3/kg (cubic meters per kilogram) | highest | 1.38×10^-8 m^3/kg (cubic meters per kilogram) (calcium) | lowest | -1.26×10^-8 m^3/kg (cubic meters per kilogram) (beryllium) | distribution | molar magnetic susceptibility | median | 1.68×10^-10 m^3/mol (cubic meters per mole) | highest | 1.552×10^-9 m^3/mol (cubic meters per mole) (barium) | lowest | -1.136×10^-10 m^3/mol (cubic meters per mole) (beryllium) | distribution | work function | all | 2.52 eV | 2.59 eV | 2.87 eV | 3.66 eV | 4.98 eV
Reactivity
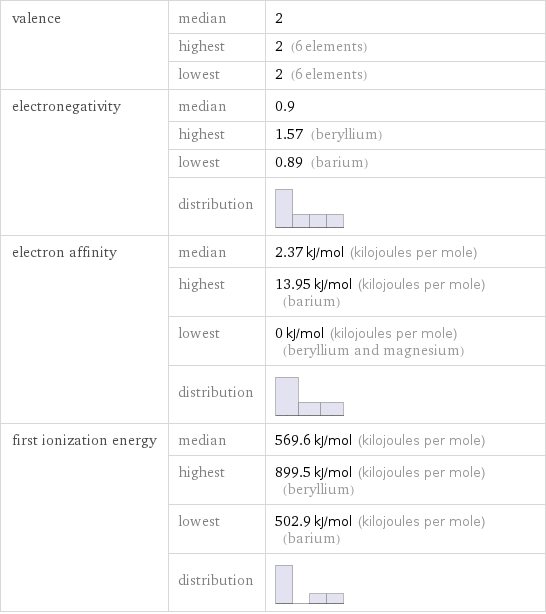
valence | median | 2 | highest | 2 (6 elements) | lowest | 2 (6 elements) electronegativity | median | 0.9 | highest | 1.57 (beryllium) | lowest | 0.89 (barium) | distribution | electron affinity | median | 2.37 kJ/mol (kilojoules per mole) | highest | 13.95 kJ/mol (kilojoules per mole) (barium) | lowest | 0 kJ/mol (kilojoules per mole) (beryllium and magnesium) | distribution | first ionization energy | median | 569.6 kJ/mol (kilojoules per mole) | highest | 899.5 kJ/mol (kilojoules per mole) (beryllium) | lowest | 502.9 kJ/mol (kilojoules per mole) (barium) | distribution |