Input interpretation

polar protic solvents | molar heat of vaporization
Summary
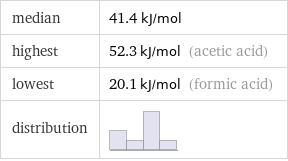
median | 41.4 kJ/mol highest | 52.3 kJ/mol (acetic acid) lowest | 20.1 kJ/mol (formic acid) distribution |
Units

Ranked values
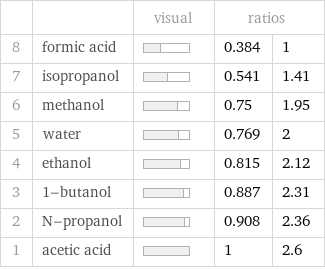
| | visual | ratios | 8 | formic acid | | 0.384 | 1 7 | isopropanol | | 0.541 | 1.41 6 | methanol | | 0.75 | 1.95 5 | water | | 0.769 | 2 4 | ethanol | | 0.815 | 2.12 3 | 1-butanol | | 0.887 | 2.31 2 | N-propanol | | 0.908 | 2.36 1 | acetic acid | | 1 | 2.6
Molar heat of vaporization rankings
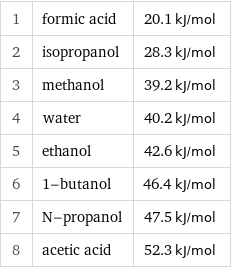
1 | formic acid | 20.1 kJ/mol 2 | isopropanol | 28.3 kJ/mol 3 | methanol | 39.2 kJ/mol 4 | water | 40.2 kJ/mol 5 | ethanol | 42.6 kJ/mol 6 | 1-butanol | 46.4 kJ/mol 7 | N-propanol | 47.5 kJ/mol 8 | acetic acid | 52.3 kJ/mol
Unit conversions for median molar heat of vaporization 41.4 kJ/mol

0.0414 MJ/mol (megajoules per mole)
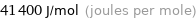
41400 J/mol (joules per mole)

9.89 kcal/mol (thermochemical kilocalories per mole)
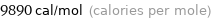
9890 cal/mol (calories per mole)

0.429 eV (molar electronvolts)