Input interpretation
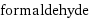
formaldehyde
Chemical names and formulas

formula | HCHO Hill formula | CH_2O name | formaldehyde alternate names | formalin | formol | superlysoform mass fractions | C (carbon) 40% | H (hydrogen) 6.71% | O (oxygen) 53.3%
Lewis structure

Draw the Lewis structure of formaldehyde. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), and oxygen (n_O, val = 6) atoms: n_C, val + 2 n_H, val + n_O, val = 12 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), and oxygen (n_O, full = 8): n_C, full + 2 n_H, full + n_O, full = 20 Subtracting these two numbers shows that 20 - 12 = 8 bonding electrons are needed. Each bond has two electrons, so in addition to the 3 bonds already present in the diagram add 1 bond. To minimize formal charge oxygen wants 2 bonds and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 1 bond by pairing electrons between adjacent highlighted atoms: Answer: | |
3D structure

3D structure
Basic properties
molar mass | 30.026 g/mol phase | gas (at STP) melting point | -92 °C boiling point | -19.1 °C density | 1.09 g/cm^3 (at 25 °C) solubility in water | miscible
Units

Hydrophobicity and permeability properties

experimental LogP hydrophobicity | 0.35 predicted LogP hydrophobicity | -0.68 predicted LogS | 0.82
Basic drug properties

approval status | experimental | small molecule drug categories | disinfectant | fixative dosage forms | topical: solution

brand names | formalin | formol
Gas properties (at STP)
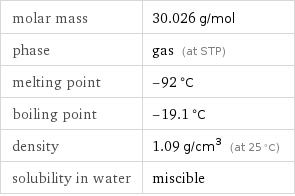
density | 1.09 g/cm^3 (at 25 °C) vapor density | 1.03 (relative to air) molar volume | 27.55 cm^3/mol refractive index | 1.38
Units

Thermodynamic properties
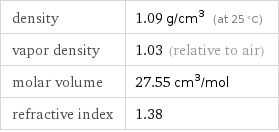
specific free energy of formation Δ_fG° | gas | -3.414 kJ/g molar free energy of formation Δ_fG° | gas | -102.5 kJ/mol specific heat of formation Δ_fH° | gas | -3.617 kJ/g molar heat of formation Δ_fH° | gas | -108.6 kJ/mol molar heat of vaporization | 23.3 kJ/mol | specific heat of vaporization | 0.776 kJ/g | critical temperature | 410.3 K | (at STP)
Chemical identifiers
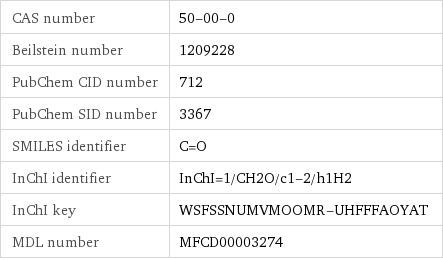
CAS number | 50-00-0 Beilstein number | 1209228 PubChem CID number | 712 PubChem SID number | 3367 SMILES identifier | C=O InChI identifier | InChI=1/CH2O/c1-2/h1H2 InChI key | WSFSSNUMVMOOMR-UHFFFAOYAT MDL number | MFCD00003274
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 2 NFPA reactivity rating | 0
Safety properties
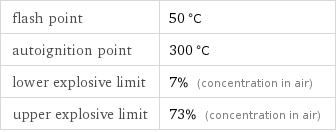
flash point | 50 °C autoignition point | 300 °C lower explosive limit | 7% (concentration in air) upper explosive limit | 73% (concentration in air)
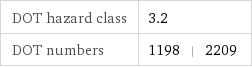
DOT hazard class | 3.2 DOT numbers | 1198 | 2209
Toxicity properties
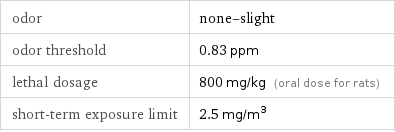
odor | none-slight odor threshold | 0.83 ppm lethal dosage | 800 mg/kg (oral dose for rats) short-term exposure limit | 2.5 mg/m^3

probable lethal dose for man | 600 mL (milliliters) long-term exposure limit | 2.5 mg/m^3 (over 8 hours) RTECS classes | agricultural chemical and pesticide | tumorigen | mutagen | reproductive effector | human data | primary irritant