Input interpretation

group 7 elements | specific heat at STP
Results

manganese | 479 J/(kg K) (joules per kilogram kelvin difference) technetium | 63 J/(kg K) (joules per kilogram kelvin difference) rhenium | 137 J/(kg K) (joules per kilogram kelvin difference) bohrium | (unknown)

group 7 elements | specific heat at STP | 137 J/(kg K) (joules per kilogram kelvin difference) (median) (based on 3 values; 1 unavailable)
Ranked values

| | visual | ratios | 3 | technetium | | 0.13 | 1 2 | rhenium | | 0.286 | 2.2 1 | manganese | | 1 | 7.6
Unit conversions for median specific heat at STP 137 J/(kg K)
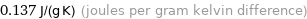
0.137 J/(g K) (joules per gram kelvin difference)
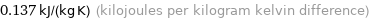
0.137 kJ/(kg K) (kilojoules per kilogram kelvin difference)
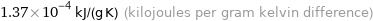
1.37×10^-4 kJ/(g K) (kilojoules per gram kelvin difference)

137 J/(kg °C) (joules per kilogram degree Celsius difference)
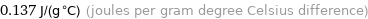
0.137 J/(g °C) (joules per gram degree Celsius difference)

0.0327 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons for median specific heat at STP 137 J/(kg K)
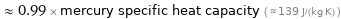
≈ 0.99 × mercury specific heat capacity ( ≈ 139 J/(kg K) )
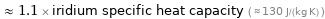
≈ 1.1 × iridium specific heat capacity ( ≈ 130 J/(kg K) )
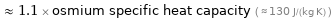
≈ 1.1 × osmium specific heat capacity ( ≈ 130 J/(kg K) )
Thermodynamic properties
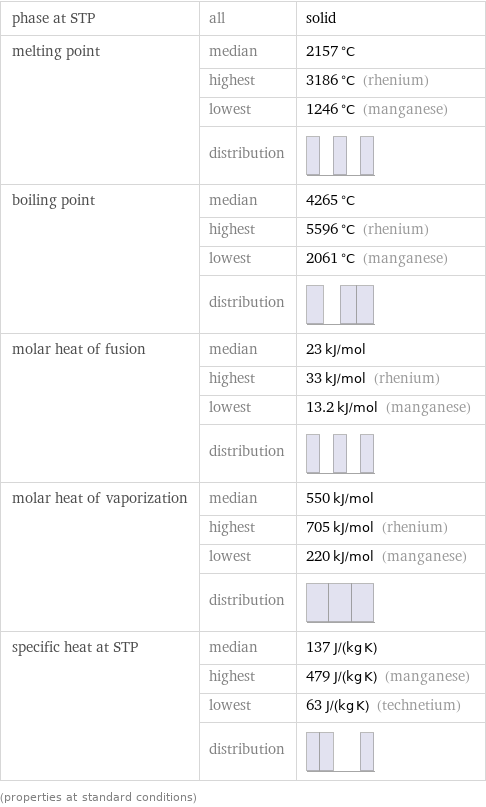
phase at STP | all | solid melting point | median | 2157 °C | highest | 3186 °C (rhenium) | lowest | 1246 °C (manganese) | distribution | boiling point | median | 4265 °C | highest | 5596 °C (rhenium) | lowest | 2061 °C (manganese) | distribution | molar heat of fusion | median | 23 kJ/mol | highest | 33 kJ/mol (rhenium) | lowest | 13.2 kJ/mol (manganese) | distribution | molar heat of vaporization | median | 550 kJ/mol | highest | 705 kJ/mol (rhenium) | lowest | 220 kJ/mol (manganese) | distribution | specific heat at STP | median | 137 J/(kg K) | highest | 479 J/(kg K) (manganese) | lowest | 63 J/(kg K) (technetium) | distribution | (properties at standard conditions)