Input interpretation
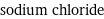
sodium chloride
Chemical names and formulas
formula | NaCl Hill formula | ClNa name | sodium chloride alternate names | common salt | rock salt | salt | table salt mass fractions | Cl (chlorine) 60.7% | Na (sodium) 39.3%
Structure diagram

Structure diagram
Basic properties
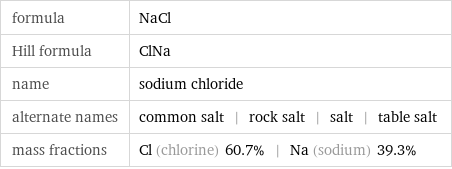
molar mass | 58.44 g/mol phase | solid (at STP) melting point | 801 °C boiling point | 1413 °C density | 2.16 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)
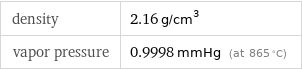
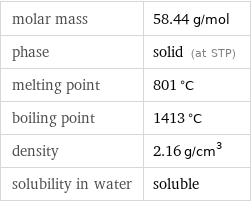
density | 2.16 g/cm^3 vapor pressure | 0.9998 mmHg (at 865 °C)
Units

Thermodynamic properties
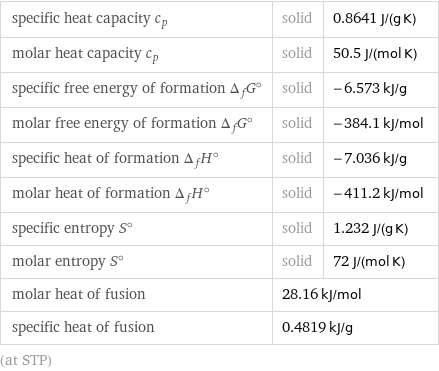
specific heat capacity c_p | solid | 0.8641 J/(g K) molar heat capacity c_p | solid | 50.5 J/(mol K) specific free energy of formation Δ_fG° | solid | -6.573 kJ/g molar free energy of formation Δ_fG° | solid | -384.1 kJ/mol specific heat of formation Δ_fH° | solid | -7.036 kJ/g molar heat of formation Δ_fH° | solid | -411.2 kJ/mol specific entropy S° | solid | 1.232 J/(g K) molar entropy S° | solid | 72 J/(mol K) molar heat of fusion | 28.16 kJ/mol | specific heat of fusion | 0.4819 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7647-14-5 Beilstein number | 3534976 PubChem CID number | 5234 PubChem SID number | 24852266 SMILES identifier | [Na+].[Cl-] InChI identifier | InChI=1/ClH.Na/h1H;/q;+1/p-1/fCl.Na/h1h;/q-1;m RTECS number | VZ4725000 MDL number | MFCD00003477](../image_source/63daa1629b934d3f7409bb8b9d5b96bf.png)
CAS number | 7647-14-5 Beilstein number | 3534976 PubChem CID number | 5234 PubChem SID number | 24852266 SMILES identifier | [Na+].[Cl-] InChI identifier | InChI=1/ClH.Na/h1H;/q;+1/p-1/fCl.Na/h1h;/q-1;m RTECS number | VZ4725000 MDL number | MFCD00003477
NFPA label

NFPA label

NFPA health rating | 1 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties
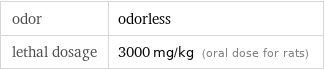
odor | odorless lethal dosage | 3000 mg/kg (oral dose for rats)

probable lethal dose for man | 600 mL (milliliters) RTECS classes | agricultural chemical and pesticide | mutagen | reproductive effector | human data | primary irritant
Ion equivalents

Na^+ (sodium cation) | 1 Cl^- (chloride anion) | 1