Input interpretation

vanadyl chloride | potassium tetracyanoplatinate(II) trihydrate
Chemical names and formulas
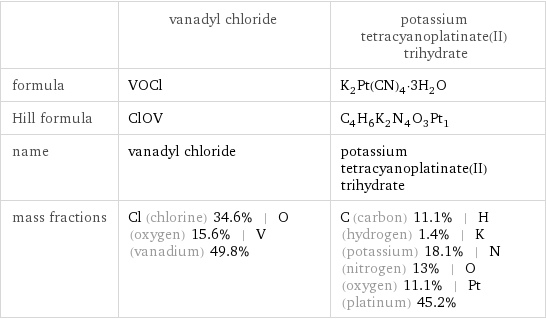
| vanadyl chloride | potassium tetracyanoplatinate(II) trihydrate formula | VOCl | K_2Pt(CN)_4·3H_2O Hill formula | ClOV | C_4H_6K_2N_4O_3Pt_1 name | vanadyl chloride | potassium tetracyanoplatinate(II) trihydrate mass fractions | Cl (chlorine) 34.6% | O (oxygen) 15.6% | V (vanadium) 49.8% | C (carbon) 11.1% | H (hydrogen) 1.4% | K (potassium) 18.1% | N (nitrogen) 13% | O (oxygen) 11.1% | Pt (platinum) 45.2%
Basic properties
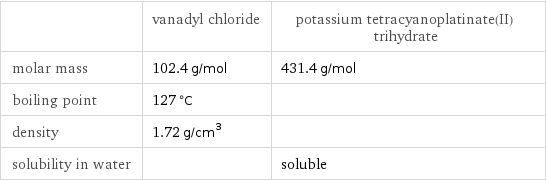
| vanadyl chloride | potassium tetracyanoplatinate(II) trihydrate molar mass | 102.4 g/mol | 431.4 g/mol boiling point | 127 °C | density | 1.72 g/cm^3 | solubility in water | | soluble
Units
