Input interpretation
carbon monoxide
Chemical names and formulas
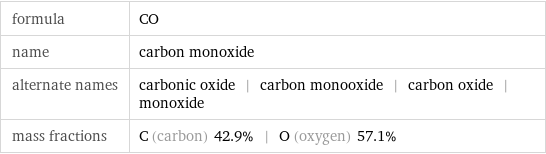
formula | CO name | carbon monoxide alternate names | carbonic oxide | carbon monooxide | carbon oxide | monoxide mass fractions | C (carbon) 42.9% | O (oxygen) 57.1%
Lewis structure
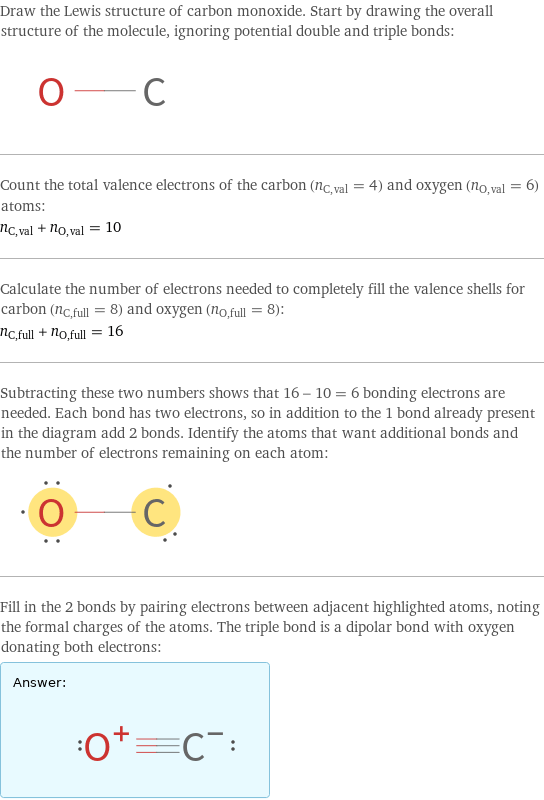
Draw the Lewis structure of carbon monoxide. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4) and oxygen (n_O, val = 6) atoms: n_C, val + n_O, val = 10 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8) and oxygen (n_O, full = 8): n_C, full + n_O, full = 16 Subtracting these two numbers shows that 16 - 10 = 6 bonding electrons are needed. Each bond has two electrons, so in addition to the 1 bond already present in the diagram add 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 2 bonds by pairing electrons between adjacent highlighted atoms, noting the formal charges of the atoms. The triple bond is a dipolar bond with oxygen donating both electrons: Answer: | |
3D structure

3D structure
Basic properties
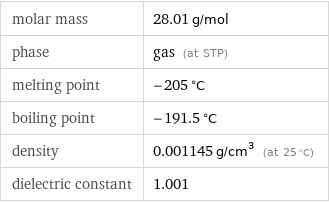
molar mass | 28.01 g/mol phase | gas (at STP) melting point | -205 °C boiling point | -191.5 °C density | 0.001145 g/cm^3 (at 25 °C) dielectric constant | 1.001
Gas properties (at STP)
density | 0.001145 g/cm^3 (at 25 °C) vapor density | 0.97 (relative to air) molar volume | 24460 cm^3/mol dynamic viscosity | 1.772×10^-5 Pa s (at 25 °C)
Units
Thermodynamic properties
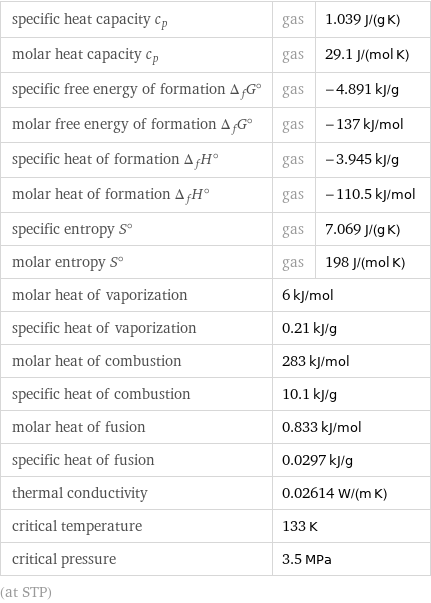
specific heat capacity c_p | gas | 1.039 J/(g K) molar heat capacity c_p | gas | 29.1 J/(mol K) specific free energy of formation Δ_fG° | gas | -4.891 kJ/g molar free energy of formation Δ_fG° | gas | -137 kJ/mol specific heat of formation Δ_fH° | gas | -3.945 kJ/g molar heat of formation Δ_fH° | gas | -110.5 kJ/mol specific entropy S° | gas | 7.069 J/(g K) molar entropy S° | gas | 198 J/(mol K) molar heat of vaporization | 6 kJ/mol | specific heat of vaporization | 0.21 kJ/g | molar heat of combustion | 283 kJ/mol | specific heat of combustion | 10.1 kJ/g | molar heat of fusion | 0.833 kJ/mol | specific heat of fusion | 0.0297 kJ/g | thermal conductivity | 0.02614 W/(m K) | critical temperature | 133 K | critical pressure | 3.5 MPa | (at STP)
Phase diagram
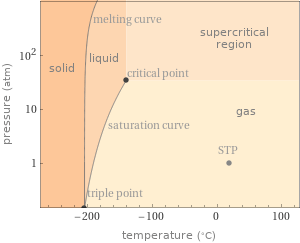
Phase diagram
Units

Chemical identifiers
![CAS number | 630-08-0 Beilstein number | 3587264 PubChem CID number | 281 PubChem SID number | 24857760 SMILES identifier | [C-]#[O+] InChI identifier | InChI=1/CO/c1-2 RTECS number | FG3500000 MDL number | MFCD00011320](../image_source/96bd625d779cd2288df917540b7eebf4.png)
CAS number | 630-08-0 Beilstein number | 3587264 PubChem CID number | 281 PubChem SID number | 24857760 SMILES identifier | [C-]#[O+] InChI identifier | InChI=1/CO/c1-2 RTECS number | FG3500000 MDL number | MFCD00011320
NFPA label

NFPA label

NFPA health rating | 4 NFPA fire rating | 2 NFPA reactivity rating | 0