Input interpretation
magnesium fluoride
Chemical names and formulas

formula | MgF_2 Hill formula | F_2Mg name | magnesium fluoride IUPAC name | magnesium difluoride alternate names | afluon | azelastine | magnesium difluoride mass fractions | F (fluorine) 61% | Mg (magnesium) 39%
Structure diagram
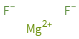
Structure diagram
Basic properties
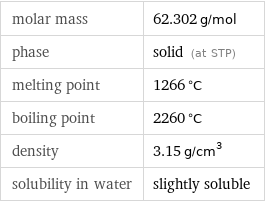
molar mass | 62.302 g/mol phase | solid (at STP) melting point | 1266 °C boiling point | 2260 °C density | 3.15 g/cm^3 solubility in water | slightly soluble
Units

Hydrophobicity and permeability properties

experimental LogP hydrophobicity | 4.9 predicted LogP hydrophobicity | 3.81 predicted LogS | -4.62
Basic drug properties

approval status | approved | small molecule drug categories | anti-allergic agent | non-steroidal anti-inflammatory agent | bronchodilator agent | histamine h1 antagonist | non-sedating histamine h1 antagonist | lipoxygenase inhibitor | platelet aggregation inhibitor dosage forms | ophthalmic: solution / drops

brand names | astelin | optivar
Solid properties (at STP)

density | 3.15 g/cm^3
Units

Thermodynamic properties
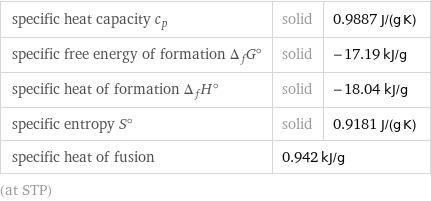
specific heat capacity c_p | solid | 0.9887 J/(g K) specific free energy of formation Δ_fG° | solid | -17.19 kJ/g specific heat of formation Δ_fH° | solid | -18.04 kJ/g specific entropy S° | solid | 0.9181 J/(g K) specific heat of fusion | 0.942 kJ/g | (at STP)
Units

Chemical identifiers
![CAS number | 7783-40-6 PubChem CID number | 24546 PubChem SID number | 9970 SMILES identifier | [F-].[F-].[Mg+2]](../image_source/7e1f750f25770a7fe7f0573633bba9ef.png)
CAS number | 7783-40-6 PubChem CID number | 24546 PubChem SID number | 9970 SMILES identifier | [F-].[F-].[Mg+2]
Toxicity properties

RTECS classes | other
Ion equivalents

Mg^(2+) (magnesium cation) | 1 F^- (fluoride anion) | 2