Input interpretation
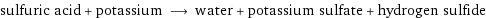
sulfuric acid + potassium ⟶ water + potassium sulfate + hydrogen sulfide
Balanced equation

Balance the chemical equation algebraically: + ⟶ + + Add stoichiometric coefficients, c_i, to the reactants and products: c_1 + c_2 ⟶ c_3 + c_4 + c_5 Set the number of atoms in the reactants equal to the number of atoms in the products for H, O, S and K: H: | 2 c_1 = 2 c_3 + 2 c_5 O: | 4 c_1 = c_3 + 4 c_4 S: | c_1 = c_4 + c_5 K: | c_2 = 2 c_4 Since the coefficients are relative quantities and underdetermined, choose a coefficient to set arbitrarily. To keep the coefficients small, the arbitrary value is ordinarily one. For instance, set c_5 = 1 and solve the system of equations for the remaining coefficients: c_1 = 5 c_2 = 8 c_3 = 4 c_4 = 4 c_5 = 1 Substitute the coefficients into the chemical reaction to obtain the balanced equation: Answer: | | 5 + 8 ⟶ 4 + 4 +
Structures
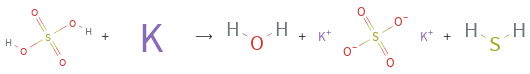
+ ⟶ + +
Names
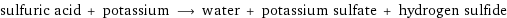
sulfuric acid + potassium ⟶ water + potassium sulfate + hydrogen sulfide
Chemical names and formulas
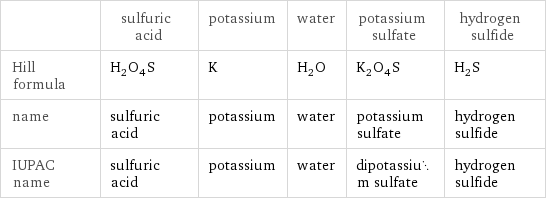
| sulfuric acid | potassium | water | potassium sulfate | hydrogen sulfide Hill formula | H_2O_4S | K | H_2O | K_2O_4S | H_2S name | sulfuric acid | potassium | water | potassium sulfate | hydrogen sulfide IUPAC name | sulfuric acid | potassium | water | dipotassium sulfate | hydrogen sulfide