Input interpretation
iron(III) oxide
Chemical names and formulas

formula | Fe_2O_3 name | iron(III) oxide alternate names | diiron trioxide | ferric oxide | haematite | hematite | iron sesquioxide | oxo-(oxoferriooxy)iron | red iron oxide | specularite mass fractions | Fe (iron) 69.9% | O (oxygen) 30.1%
Structure diagram
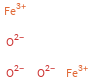
Structure diagram
Basic properties
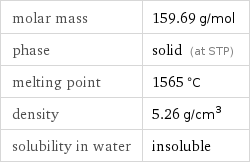
molar mass | 159.69 g/mol phase | solid (at STP) melting point | 1565 °C density | 5.26 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)

density | 5.26 g/cm^3
Units

Thermodynamic properties
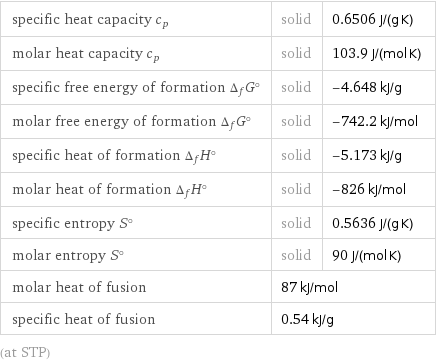
specific heat capacity c_p | solid | 0.6506 J/(g K) molar heat capacity c_p | solid | 103.9 J/(mol K) specific free energy of formation Δ_fG° | solid | -4.648 kJ/g molar free energy of formation Δ_fG° | solid | -742.2 kJ/mol specific heat of formation Δ_fH° | solid | -5.173 kJ/g molar heat of formation Δ_fH° | solid | -826 kJ/mol specific entropy S° | solid | 0.5636 J/(g K) molar entropy S° | solid | 90 J/(mol K) molar heat of fusion | 87 kJ/mol | specific heat of fusion | 0.54 kJ/g | (at STP)
Chemical identifiers
![CAS number | 1309-37-1 SMILES identifier | [Fe+3].[Fe+3].[O-2].[O-2].[O-2] EU number | 215-275-4 Gmelin number | -81 RTECS number | NO7400000 MDL number | MFCD00011008](../image_source/a9646996ab40a849897091d071b472de.png)
CAS number | 1309-37-1 SMILES identifier | [Fe+3].[Fe+3].[O-2].[O-2].[O-2] EU number | 215-275-4 Gmelin number | -81 RTECS number | NO7400000 MDL number | MFCD00011008
Toxicity properties

odor | odorless

RTECS classes | tumorigen